Life Science
... of Protons in the nucleus Atomic Mass – The sum of protons & neutrons in an nucleus Isotope – types of an element w/ different #’s of neutrons in the nucleus. Mole – a type of unit used to measure atom numbers: ...
... of Protons in the nucleus Atomic Mass – The sum of protons & neutrons in an nucleus Isotope – types of an element w/ different #’s of neutrons in the nucleus. Mole – a type of unit used to measure atom numbers: ...
Life Science Chapter 1 Part 2 Chemical Compounds in Cells
... of Protons in the nucleus Atomic Mass – The sum of protons & neutrons in an nucleus Isotope – types of an element w/ different #’s of neutrons in the nucleus. Mole – a type of unit used to measure atom numbers: ...
... of Protons in the nucleus Atomic Mass – The sum of protons & neutrons in an nucleus Isotope – types of an element w/ different #’s of neutrons in the nucleus. Mole – a type of unit used to measure atom numbers: ...
Donald C. Cox Seminar Series in Microbiology Presents:
... !Soil represents a massive reservoir of active carbon and climate models vary dramatically in predicting how this carbon will respond to climate change over the coming century. A major cause of uncertainty is that we really still don’t understand the microorganisms that dominate the soil carbon cycl ...
... !Soil represents a massive reservoir of active carbon and climate models vary dramatically in predicting how this carbon will respond to climate change over the coming century. A major cause of uncertainty is that we really still don’t understand the microorganisms that dominate the soil carbon cycl ...
Nuclear Chemistry I: Radioactivity Reading: Moore chapter 20
... Calculate nuclear binding energy for a particular isotope; calculate half-life from activity; use half-life to find the time required for an isotope to decay to a particular activity. Lecture Topics: I. Nuclear Chemistry - definitions Nuclear Chemistry is closely connected with the study of radioact ...
... Calculate nuclear binding energy for a particular isotope; calculate half-life from activity; use half-life to find the time required for an isotope to decay to a particular activity. Lecture Topics: I. Nuclear Chemistry - definitions Nuclear Chemistry is closely connected with the study of radioact ...
Chemistry Honors Unit 2 Study Guide Atomic Theory Mr. Brown Use
... Law of Definite Proportions/Composition = Chemical compounds always contain the same elements in exactly the same proportions by mass regardless of the amount or source of the sample. EX. NaCl always contain 39.34% by mass of Na and 60.66% by mass of Cl. Law of Multiple Proportions = If two or more ...
... Law of Definite Proportions/Composition = Chemical compounds always contain the same elements in exactly the same proportions by mass regardless of the amount or source of the sample. EX. NaCl always contain 39.34% by mass of Na and 60.66% by mass of Cl. Law of Multiple Proportions = If two or more ...
atoms - Trinity Regional School
... Mixture – 2 or more elements, compounds, or both physically combined. homogenous or heterogeneous ...
... Mixture – 2 or more elements, compounds, or both physically combined. homogenous or heterogeneous ...
Chemistry Review: Antoine Lavoisier (1743
... Isotopes: atoms of the same element that differs in mass. Ex there are 3 isotopes of hydrogen, with mass numbers of 1, 2 and 3. These atoms have different masses because they have different numbers of neutrons. However, since they have the same number of protons, they are the same element. Most el ...
... Isotopes: atoms of the same element that differs in mass. Ex there are 3 isotopes of hydrogen, with mass numbers of 1, 2 and 3. These atoms have different masses because they have different numbers of neutrons. However, since they have the same number of protons, they are the same element. Most el ...
Biochemistry
... up chemical __________by lowering the activation ___________ End with –________ and their name is related to the compound they act upon Example: lactase – speeds up the reaction that breaks down the disaccharide lactose into the monosaccharides galactose and ...
... up chemical __________by lowering the activation ___________ End with –________ and their name is related to the compound they act upon Example: lactase – speeds up the reaction that breaks down the disaccharide lactose into the monosaccharides galactose and ...
subatomic particles
... Carbon-12 atom and dividing by the total number of particles. - Atomic number: the number of protons in an atom. - Mass number: the sum of the protons and neutrons in an atom. - Isotopes: atoms of the same element with different masses. They have the same number of protons and electrons but greater ...
... Carbon-12 atom and dividing by the total number of particles. - Atomic number: the number of protons in an atom. - Mass number: the sum of the protons and neutrons in an atom. - Isotopes: atoms of the same element with different masses. They have the same number of protons and electrons but greater ...
Atomic Timeline
... Isotopes – atoms of an element that have the same number of protons but different numbers of neutrons. This change in neutrons is reflected in the Mass Number ...
... Isotopes – atoms of an element that have the same number of protons but different numbers of neutrons. This change in neutrons is reflected in the Mass Number ...
•What makes up an atom? Draw an atom
... • Element - Pure substance that can’t be broken into other types of matter • Each element has its own symbol and specific number of protons ...
... • Element - Pure substance that can’t be broken into other types of matter • Each element has its own symbol and specific number of protons ...
Ch. 6 Vocabulary
... Catalyst – a substance that lowers the activation energy needed to start a chemical reaction Enzymes – a protein and biological catalysts that speed up the rate of chemical reactions in biological processes ...
... Catalyst – a substance that lowers the activation energy needed to start a chemical reaction Enzymes – a protein and biological catalysts that speed up the rate of chemical reactions in biological processes ...
PowerPoint - Models of the Atom - A Historical Perspective
... – Often, at least one isotope is unstable.It breaks down, releasing radioactivity.These types of isotopes are called radioisotopes ...
... – Often, at least one isotope is unstable.It breaks down, releasing radioactivity.These types of isotopes are called radioisotopes ...
Study Guide - Honors Chemistry
... one nucleus is broken into multiple (2 in this case) nuclei by force (an alpha particle is used to break it up) one nucleus is broken into multiple (2 in this case) nuclei on its own. No force is needed. one nucleus is transformed into another nucleus by bombarding a particle into it. A particle may ...
... one nucleus is broken into multiple (2 in this case) nuclei by force (an alpha particle is used to break it up) one nucleus is broken into multiple (2 in this case) nuclei on its own. No force is needed. one nucleus is transformed into another nucleus by bombarding a particle into it. A particle may ...
CHEM 101 Dual Enrollment HW4 Question 1 of 12 Dalton`s
... Question 1 of 12 Dalton's postulates, also known as Dalton's atomic theory, include some proposals that have been updated or changed due to new discoveries. Which of the following statements were parts of Dalton's original atomic theory? Select all that apply. Atoms of the same element have the same ...
... Question 1 of 12 Dalton's postulates, also known as Dalton's atomic theory, include some proposals that have been updated or changed due to new discoveries. Which of the following statements were parts of Dalton's original atomic theory? Select all that apply. Atoms of the same element have the same ...
1st Term Review
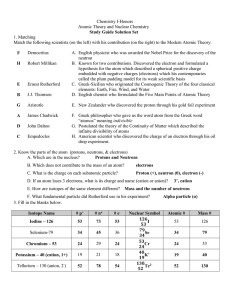
... 13. What is the mass of grams of 0.500 moles of Au? 14. Based on the gold foil experiment, what did Rutherford conclude about the atom? 15. An atom of chromium-60 contains how many protons, neutron and electrons? 16. What is the difference between a compound and an element? 17. What is the electron ...
... 13. What is the mass of grams of 0.500 moles of Au? 14. Based on the gold foil experiment, what did Rutherford conclude about the atom? 15. An atom of chromium-60 contains how many protons, neutron and electrons? 16. What is the difference between a compound and an element? 17. What is the electron ...
Chapter 2-1 The Nature of Matter
... The necessity for a close, if brief, fit between enzyme and substrate explains the phenomenon of competitive inhibition. One of the enzymes needed for the release of energy within the cell is succinic dehydrogenase. It catalyzes the oxidation (by the removal of two hydrogen atoms) of succinic acid ( ...
... The necessity for a close, if brief, fit between enzyme and substrate explains the phenomenon of competitive inhibition. One of the enzymes needed for the release of energy within the cell is succinic dehydrogenase. It catalyzes the oxidation (by the removal of two hydrogen atoms) of succinic acid ( ...
II. Radioactive Decay
... Isotopes - atoms of the same element with the same number of protons but different numbers of neutrons Radioisotopes – isotope with an unstable nucleus that emits radiation to become a more stable nucleus ...
... Isotopes - atoms of the same element with the same number of protons but different numbers of neutrons Radioisotopes – isotope with an unstable nucleus that emits radiation to become a more stable nucleus ...
Atoms: The building blocks of matter
... A chemical compound contains the same elements in exactly the same proportions by mass regardless of the size of the sample or source of the compound. ...
... A chemical compound contains the same elements in exactly the same proportions by mass regardless of the size of the sample or source of the compound. ...
Study Notes
... Strategies for Studying: • DO your study notes!! • Look at the figures (pictures) and read the captions. • Read the chapter summary. • Practice with the chapter review. • Review pages in Reading-Note Taking Guide (workbook) Location in atom ...
... Strategies for Studying: • DO your study notes!! • Look at the figures (pictures) and read the captions. • Read the chapter summary. • Practice with the chapter review. • Review pages in Reading-Note Taking Guide (workbook) Location in atom ...
General CHemistry Unit 2 Homework Notes
... A neutron has no charge and a relative mass of one. TOPIC TWO: COMPOUNDS & BONDING (PAGE 2) Subscripts in a chemical formula represent the relative number of each type of atom. The subscript always follows the symbol for the element. Example: In a water molecule, H2O, there are 2 hydrogen atoms and ...
... A neutron has no charge and a relative mass of one. TOPIC TWO: COMPOUNDS & BONDING (PAGE 2) Subscripts in a chemical formula represent the relative number of each type of atom. The subscript always follows the symbol for the element. Example: In a water molecule, H2O, there are 2 hydrogen atoms and ...
Isotopic labeling

Isotopic labeling (or isotopic labelling) is a technique used to track the passage of an isotope, or an atom with a variation, through a reaction, metabolic pathway, or cell. The reactant is 'labeled' by replacing specific atoms by their isotope. The reactant is then allowed to undergo the reaction. The position of the isotopes in the products is measured to determine the sequence the isotopic atom followed in the reaction or the cell's metabolic pathway. The nuclides used in isotopic labeling may be stable nuclides or radionuclides. In the latter case, the labeling is called radiolabeling.In isotopic labeling, there are multiple ways to detect the presence of labeling isotopes; through their mass, vibrational mode, or radioactive decay. Mass spectrometry detects the difference in an isotope's mass, while infrared spectroscopy detects the difference in the isotope's vibrational modes. Nuclear magnetic resonance detects atoms with different gyromagnetic ratios. The radioactive decay can be detected through an ionization chamber or autoradiographs of gels.An example of the use of isotopic labeling is the study of phenol (C6H5OH) in water by replacing common hydrogen (protium) with deuterium (deuterium labeling). Upon adding phenol to deuterated water (water containing D2O in addition to the usual H2O), the substitution of deuterium for the hydrogen is observed in phenol's hydroxyl group (resulting in C6H5OD), indicating that phenol readily undergoes hydrogen-exchange reactions with water. Only the hydroxyl group was affected, indicating that the other 5 hydrogen atoms did not participate in these exchange reactions.