Unit 2 Test Review - Liberty High School
... Complete the following problems to help prepare you for you Unit 2 Test. You more than likely will want to answer these questions on a separate piece of paper (unless you can write microscopically). In addition to these problems, review your notes, assignments (#29-44), labs, and chapter 3 & 4 in yo ...
... Complete the following problems to help prepare you for you Unit 2 Test. You more than likely will want to answer these questions on a separate piece of paper (unless you can write microscopically). In addition to these problems, review your notes, assignments (#29-44), labs, and chapter 3 & 4 in yo ...
Protons neutrons electrons Charge Positive neutral negative Mass
... • Gold foil experiment • Planetary model; nuclear atom ...
... • Gold foil experiment • Planetary model; nuclear atom ...
Atomic Number… - Taylor County Schools
... • In the late 1890s, scientists noticed some substances spontaneously emitted radiation, a process they called radioactivity. • The rays and particles emitted are called radiation. • A reaction that involves a change in an atom's nucleus is called a nuclear reaction. ...
... • In the late 1890s, scientists noticed some substances spontaneously emitted radiation, a process they called radioactivity. • The rays and particles emitted are called radiation. • A reaction that involves a change in an atom's nucleus is called a nuclear reaction. ...
Key
... Show this formation in three steps and show valence electrons in each step: show all atoms; show how ions form by movement of electrons; show how ions are attracted to form a compound. 2 Na atoms, each with a single ve- and 1 O atom with 6 ve Nas’ ve- move to oxygen creating atoms: Na+1 with no v ...
... Show this formation in three steps and show valence electrons in each step: show all atoms; show how ions form by movement of electrons; show how ions are attracted to form a compound. 2 Na atoms, each with a single ve- and 1 O atom with 6 ve Nas’ ve- move to oxygen creating atoms: Na+1 with no v ...
Document
... • Per unit volume, an atom bomb may be millions or billions of times more powerful than TNT. • Nuclear reactions (rxn) occur: neutrons r fired @ closely packed atoms w/ heavy nuclei (uranium or plutonium isotopes). ...
... • Per unit volume, an atom bomb may be millions or billions of times more powerful than TNT. • Nuclear reactions (rxn) occur: neutrons r fired @ closely packed atoms w/ heavy nuclei (uranium or plutonium isotopes). ...
Nuclear Chemistry 9
... Nuclear reactions Total number of atomic numbers and the total mass numbers must be equal on both sides of the equation. Examples: ...
... Nuclear reactions Total number of atomic numbers and the total mass numbers must be equal on both sides of the equation. Examples: ...
Atomic Structure Scientists
... • Compounds consist of atoms of different elements combined together. • Compounds have constant composition because they contain a fixed ratio of atoms. • Chemical reactions involve the rearrangement of combinations of those atoms. ...
... • Compounds consist of atoms of different elements combined together. • Compounds have constant composition because they contain a fixed ratio of atoms. • Chemical reactions involve the rearrangement of combinations of those atoms. ...
The ATOM - Aarmstrongchem
... be created or destroyed in ordinary chemical or physical changes Law of definite composition: a chemical compound contains the same elements in exactly the same proportions regardless of the size of the sample or the source ...
... be created or destroyed in ordinary chemical or physical changes Law of definite composition: a chemical compound contains the same elements in exactly the same proportions regardless of the size of the sample or the source ...
Chemistry lecture notes
... only one stable isotope (e.g. 19F, 27Al, 31P). others may have several (e.g. 1H and 2H, the latter also being called deuterium, 12C and 13C). Molar mass is also known as Relative atomic mass (RAM) is determine by the Proportions (Mixture of the isotopes of an element). ...
... only one stable isotope (e.g. 19F, 27Al, 31P). others may have several (e.g. 1H and 2H, the latter also being called deuterium, 12C and 13C). Molar mass is also known as Relative atomic mass (RAM) is determine by the Proportions (Mixture of the isotopes of an element). ...
200
... • Q Dalton said all atoms are identical. Right or wrong… explain. • A Wrong, isotopes are the same atom with different numbers of neutrons. ...
... • Q Dalton said all atoms are identical. Right or wrong… explain. • A Wrong, isotopes are the same atom with different numbers of neutrons. ...
Chapter 2 - profpaz.com
... The mass of the atom and the percent abundance of its isotopes are measured using mass spectrometry, a technique that separates particles according to their mass. The mass-spectrum of an element (shown below) indicates the abundance of each isotope relative to the most abundant isotope (usually set ...
... The mass of the atom and the percent abundance of its isotopes are measured using mass spectrometry, a technique that separates particles according to their mass. The mass-spectrum of an element (shown below) indicates the abundance of each isotope relative to the most abundant isotope (usually set ...
File
... Vary in mass, but are all atoms of the same element because they have the same number of protons When it is important to distinguish one isotope from another, the mass number will follow the element name o Ex.: Carbon-12 ...
... Vary in mass, but are all atoms of the same element because they have the same number of protons When it is important to distinguish one isotope from another, the mass number will follow the element name o Ex.: Carbon-12 ...
Chapter 2 Notes: The Chemistry of Life
... saturated fat, unsaturated fat, nucleic acids, nucleotides, proteins, amino acids, chemical reaction, reactants, products, activation energy, catalyst, enzymes, substrates, dehydration synthesis, hydrolysis, active site, lock‐and‐key model, organic The Nature of Matter Atom: The smallest _______ ...
... saturated fat, unsaturated fat, nucleic acids, nucleotides, proteins, amino acids, chemical reaction, reactants, products, activation energy, catalyst, enzymes, substrates, dehydration synthesis, hydrolysis, active site, lock‐and‐key model, organic The Nature of Matter Atom: The smallest _______ ...
BeaniumIsotopeLab
... 1. Was the average mass of the beans a whole number or a decimal? Explain. 2. Explain any differences between the atomic mass of your BEANIUM sample and that of a different lab group. Explain why the difference would be smaller if larger samples were used. 3. What is an isotope? 4. What is the relat ...
... 1. Was the average mass of the beans a whole number or a decimal? Explain. 2. Explain any differences between the atomic mass of your BEANIUM sample and that of a different lab group. Explain why the difference would be smaller if larger samples were used. 3. What is an isotope? 4. What is the relat ...
PWE 19-2: Measuring Isotopes with a Mass Spectrometer
... O than if it contains heavier 18O, so the water that evaporated and fell on Greenland and Antarctica as snow contains an even smaller percentage of 18O than ocean water. This deficiency becomes even more pronounced for colder climates. It has been shown that a decrease of one part per million of 18O ...
... O than if it contains heavier 18O, so the water that evaporated and fell on Greenland and Antarctica as snow contains an even smaller percentage of 18O than ocean water. This deficiency becomes even more pronounced for colder climates. It has been shown that a decrease of one part per million of 18O ...
Chemical Reactions
... each type of atom is in a compound. – NaCl has 1 sodium and 1 chlorine atom – H2O has 2 hydrogen atoms and 1 oxygen atom – C6H12O6 has 6 carbon, 12 hydrogen, and 6 oxygen atoms ...
... each type of atom is in a compound. – NaCl has 1 sodium and 1 chlorine atom – H2O has 2 hydrogen atoms and 1 oxygen atom – C6H12O6 has 6 carbon, 12 hydrogen, and 6 oxygen atoms ...
Chapter 2 review key
... carbon. Carbon-12 is the most common isotope, is not radioactive, and appears on the periodic table along with the other most common isotopes. It has an atomic mass of 12, because it has 6 protons and 6 neutrons in the nucleus. Each of these has a mass of 1 atomic mass unit, which is why its atomic ...
... carbon. Carbon-12 is the most common isotope, is not radioactive, and appears on the periodic table along with the other most common isotopes. It has an atomic mass of 12, because it has 6 protons and 6 neutrons in the nucleus. Each of these has a mass of 1 atomic mass unit, which is why its atomic ...
Chapter 4 Section 3 Distinguishing Among Atoms
... • For example, carbon has two stable isotopes: – Carbon-12, which has a natural abundance of 98.89%, and – Carbon-13, which has a natural abundance of 1.11%. ...
... • For example, carbon has two stable isotopes: – Carbon-12, which has a natural abundance of 98.89%, and – Carbon-13, which has a natural abundance of 1.11%. ...
Unit 3 Review Worksheet
... c. The period 6 alkaline earth metal: _____________________________________ d. The metalloid in group 16: _____________________________________ e. The only nonmetal in group 14: _____________________________________ ...
... c. The period 6 alkaline earth metal: _____________________________________ d. The metalloid in group 16: _____________________________________ e. The only nonmetal in group 14: _____________________________________ ...
chapter 2 biochemistry
... Products are the substances formed during the reaction, on the right side of the arrow. ...
... Products are the substances formed during the reaction, on the right side of the arrow. ...
Chapter 16
... made up of exactly the same proportions of matter. (different composition in each sample) ...
... made up of exactly the same proportions of matter. (different composition in each sample) ...
Section 3-1
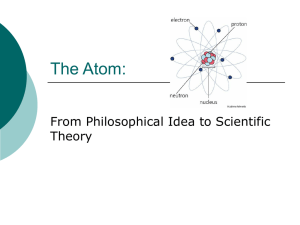
... atom in 400 BC. Atom meant indivisible Aristotle – did not believe in atoms, believed that all matter was continuous. Idea lasted 2000 years. Earth, Wind, Water, Fire, etc. ...
... atom in 400 BC. Atom meant indivisible Aristotle – did not believe in atoms, believed that all matter was continuous. Idea lasted 2000 years. Earth, Wind, Water, Fire, etc. ...
Ch 8 Carbon Chem
... A. Organic Compounds- compounds that contain Carbon. B. Hydrocarbon- compounds that contain only the elements carbon and hydrogen. C. Structural formula- shows the kind, number and arrangement of atoms in a molecule. Methane CH4 ...
... A. Organic Compounds- compounds that contain Carbon. B. Hydrocarbon- compounds that contain only the elements carbon and hydrogen. C. Structural formula- shows the kind, number and arrangement of atoms in a molecule. Methane CH4 ...
Isotopic labeling

Isotopic labeling (or isotopic labelling) is a technique used to track the passage of an isotope, or an atom with a variation, through a reaction, metabolic pathway, or cell. The reactant is 'labeled' by replacing specific atoms by their isotope. The reactant is then allowed to undergo the reaction. The position of the isotopes in the products is measured to determine the sequence the isotopic atom followed in the reaction or the cell's metabolic pathway. The nuclides used in isotopic labeling may be stable nuclides or radionuclides. In the latter case, the labeling is called radiolabeling.In isotopic labeling, there are multiple ways to detect the presence of labeling isotopes; through their mass, vibrational mode, or radioactive decay. Mass spectrometry detects the difference in an isotope's mass, while infrared spectroscopy detects the difference in the isotope's vibrational modes. Nuclear magnetic resonance detects atoms with different gyromagnetic ratios. The radioactive decay can be detected through an ionization chamber or autoradiographs of gels.An example of the use of isotopic labeling is the study of phenol (C6H5OH) in water by replacing common hydrogen (protium) with deuterium (deuterium labeling). Upon adding phenol to deuterated water (water containing D2O in addition to the usual H2O), the substitution of deuterium for the hydrogen is observed in phenol's hydroxyl group (resulting in C6H5OD), indicating that phenol readily undergoes hydrogen-exchange reactions with water. Only the hydroxyl group was affected, indicating that the other 5 hydrogen atoms did not participate in these exchange reactions.