Chapter 3.1 PPT
... different compounds are composed of the same two elements, then the ratio of the masses of the second element combined with a certain mass of the first element is always a ratio of small whole ...
... different compounds are composed of the same two elements, then the ratio of the masses of the second element combined with a certain mass of the first element is always a ratio of small whole ...
atoms, molecules, and matter (2)
... ELEMENTS – Greek theory of physical world. All earthly objects are a mixture of: 1. EARTH (bottom – center of universe) 2. WATER (water covers earth) 3. AIR (air over water) 4. FIRE (highest – at top) 5. Ether = QUINTESSENCE (Latin) – substance whose natural motion is that most symmetrical and etern ...
... ELEMENTS – Greek theory of physical world. All earthly objects are a mixture of: 1. EARTH (bottom – center of universe) 2. WATER (water covers earth) 3. AIR (air over water) 4. FIRE (highest – at top) 5. Ether = QUINTESSENCE (Latin) – substance whose natural motion is that most symmetrical and etern ...
Atoms: The Building Blocks of Matter
... • Compound: a substance that consists of two or more different types of elements. • Molecule: a substance that consists of two or more atoms. ...
... • Compound: a substance that consists of two or more different types of elements. • Molecule: a substance that consists of two or more atoms. ...
File
... Proteins are 1/more ______________________ 100s of amino acids Bent and folded due to bonding between ______________________ ...
... Proteins are 1/more ______________________ 100s of amino acids Bent and folded due to bonding between ______________________ ...
Exercise 5
... 3 = yellow; carbon 4 = green; carbon 5 = blue and carbon 6 = violet. Use orange atoms to represent phosphate groups; it is not necessary to have every single oxygen in the phosphate group represented. Go through the sequence of steps outlined on page 474 to reduce the glucose to two pyruvate molecul ...
... 3 = yellow; carbon 4 = green; carbon 5 = blue and carbon 6 = violet. Use orange atoms to represent phosphate groups; it is not necessary to have every single oxygen in the phosphate group represented. Go through the sequence of steps outlined on page 474 to reduce the glucose to two pyruvate molecul ...
The Atom.jet.2013 - Petal School District
... all available isotopes of the element, based on percent occurrence Nomenclature to distinguish isotopes: C-12 is “carbon twelve” ◦ ______ protons and ______ neutrons ...
... all available isotopes of the element, based on percent occurrence Nomenclature to distinguish isotopes: C-12 is “carbon twelve” ◦ ______ protons and ______ neutrons ...
document
... Atoms of the same element with different numbers of neutrons http://www.sci.tamucc.edu/pals/morvant/genchem/atomic/page9.htm Nuclide – general term for any isotope of any element Each isotope has a % abundance in nature Symbols for isotopes: Lithium – 6 / Lithium – 7 Isotopes differ by Number of ...
... Atoms of the same element with different numbers of neutrons http://www.sci.tamucc.edu/pals/morvant/genchem/atomic/page9.htm Nuclide – general term for any isotope of any element Each isotope has a % abundance in nature Symbols for isotopes: Lithium – 6 / Lithium – 7 Isotopes differ by Number of ...
6.1 ATOMS, ELEMENTS, and COMPOUNDS
... by covalent bonds. • Can be a single, double, or triple bond depending on number of pairs of electrons shared. 2_____________________—forms when atom gives up electrons and another receives electrons in order to become stable • Electrical attraction between two oppositely charged atoms or groups of ...
... by covalent bonds. • Can be a single, double, or triple bond depending on number of pairs of electrons shared. 2_____________________—forms when atom gives up electrons and another receives electrons in order to become stable • Electrical attraction between two oppositely charged atoms or groups of ...
Atomic Structure
... number ratios to form compounds. In chemical reactions, atoms are combined, separated or rearranged. ...
... number ratios to form compounds. In chemical reactions, atoms are combined, separated or rearranged. ...
Exercise 5
... 3 = yellow; carbon 4 = green; carbon 5 = blue and carbon 6 = violet. Use orange atoms to represent phosphate groups; it is not necessary to have every single oxygen in the phosphate group represented. Go through the sequence of steps outlined on page 474 to reduce the glucose to two pyruvate molecul ...
... 3 = yellow; carbon 4 = green; carbon 5 = blue and carbon 6 = violet. Use orange atoms to represent phosphate groups; it is not necessary to have every single oxygen in the phosphate group represented. Go through the sequence of steps outlined on page 474 to reduce the glucose to two pyruvate molecul ...
1 Atomic Mass
... Chemical Reactions- atoms from two or more different elements combine, creating new materials called compounds. Reactants (starting substances) Products (new compounds) ...
... Chemical Reactions- atoms from two or more different elements combine, creating new materials called compounds. Reactants (starting substances) Products (new compounds) ...
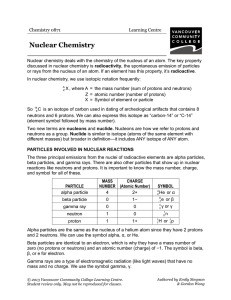
Nuclear Chemistry - VCC Library
... So 146 C is an isotope of carbon used in dating of archeological artifacts that contains 8 neutrons and 6 protons. We can also express this isotope as “carbon-14” or “C-14” (element symbol followed by mass number). Two new terms are nucleons and nuclide. Nucleons are how we refer to protons and neut ...
... So 146 C is an isotope of carbon used in dating of archeological artifacts that contains 8 neutrons and 6 protons. We can also express this isotope as “carbon-14” or “C-14” (element symbol followed by mass number). Two new terms are nucleons and nuclide. Nucleons are how we refer to protons and neut ...
Structure of the Atom Cornell Notes (pg
... example on the board). Also include any definitions like Atomic Number and Mass Number because they refer to protons in their definitions. 7. Under Neutrons write bullets of all facts from your notes about neutrons. Remember to also include definitions like Mass Number and Isotopes because they refe ...
... example on the board). Also include any definitions like Atomic Number and Mass Number because they refer to protons in their definitions. 7. Under Neutrons write bullets of all facts from your notes about neutrons. Remember to also include definitions like Mass Number and Isotopes because they refe ...
Section 4.2 The Structure of an Atom
... 13. Every atom of a given element does not have the same number of neutrons ...
... 13. Every atom of a given element does not have the same number of neutrons ...
Measurement of the half-life of
... It is well known that decay rate of radioactive nuclides is usually independent on external conditions such as chemical structures of sample materials. However, there are some exceptions in the electron capture decay and the internal conversion processes [1]. In the case of electron capture decays, ...
... It is well known that decay rate of radioactive nuclides is usually independent on external conditions such as chemical structures of sample materials. However, there are some exceptions in the electron capture decay and the internal conversion processes [1]. In the case of electron capture decays, ...
Dr Davids Essential Chemistry Definitions Bk1
... A molecule that is non-superimposable on its mirror image; such a molecule is optically active (meaning that it will rotate the plane of plane polarised light to the right or to the left). Chiral molecules frequently contain one or more asymmetric carbon atoms. Conjugate acid-base pairs: These are f ...
... A molecule that is non-superimposable on its mirror image; such a molecule is optically active (meaning that it will rotate the plane of plane polarised light to the right or to the left). Chiral molecules frequently contain one or more asymmetric carbon atoms. Conjugate acid-base pairs: These are f ...
Review Guide
... 13. What is the difference between an ionic bond and a covalent bond? 14. **Review your element names and symbols** 15. Distinguish between organic compounds and inorganic compounds. Give one example of each. 16. Why is carbon a unique element? 17. What is a hydrocarbon? 18. Draw the structural form ...
... 13. What is the difference between an ionic bond and a covalent bond? 14. **Review your element names and symbols** 15. Distinguish between organic compounds and inorganic compounds. Give one example of each. 16. Why is carbon a unique element? 17. What is a hydrocarbon? 18. Draw the structural form ...
Atomic Structure Worksheet
... Do you know why this is? After all, for our purposes, the mass of both the proton and the neutron are almost exactly 1, and in chemistry we usually ignore the mass of the electron because it is so very small. Why then, if the mass of the atom comes mainly from the protons and neutrons it contains, d ...
... Do you know why this is? After all, for our purposes, the mass of both the proton and the neutron are almost exactly 1, and in chemistry we usually ignore the mass of the electron because it is so very small. Why then, if the mass of the atom comes mainly from the protons and neutrons it contains, d ...
Structure of the atom
... __________________________________________ __________________________________________ ...
... __________________________________________ __________________________________________ ...
Electron
... Nerdy Nelda Neutron is large like Patty, but she has a boring, flat mouth and eyes with zero expression (o). Her family is very apathetic and neutral about everything. Like Patty, Nelda, and their sisters spend all their time in the arcade. ...
... Nerdy Nelda Neutron is large like Patty, but she has a boring, flat mouth and eyes with zero expression (o). Her family is very apathetic and neutral about everything. Like Patty, Nelda, and their sisters spend all their time in the arcade. ...
Atomic theory
... of a proton. Electrons are in constant motion around the nucleus of atoms. They are attracted to the opposite charge of the protons in the nucleus, but remain outside due to the energy of their motion. ...
... of a proton. Electrons are in constant motion around the nucleus of atoms. They are attracted to the opposite charge of the protons in the nucleus, but remain outside due to the energy of their motion. ...
Periodic Table
... Dalton’s Atomic Theory 1. All matter is composed of extremely small particles ...
... Dalton’s Atomic Theory 1. All matter is composed of extremely small particles ...
Quizlet Vocab Chapter 2
... negatively charged particle of an atom that orbits around the nucleus ...
... negatively charged particle of an atom that orbits around the nucleus ...
Radioactive Elements (pages 139–146)
... tracing the steps of chemical reactions and industrial processes, diagnosing and treating disease, and providing sources of energy. • Radioactive isotopes are useful for two reasons. First, radioactive isotopes change into different elements. Second, radioactive isotopes give off radiation that can ...
... tracing the steps of chemical reactions and industrial processes, diagnosing and treating disease, and providing sources of energy. • Radioactive isotopes are useful for two reasons. First, radioactive isotopes change into different elements. Second, radioactive isotopes give off radiation that can ...
ATOMS
... – States that a chemical compound contains the same elements in exactly the same proportions by mass regardless of the size of the sample or source of the compound ...
... – States that a chemical compound contains the same elements in exactly the same proportions by mass regardless of the size of the sample or source of the compound ...
Isotopic labeling

Isotopic labeling (or isotopic labelling) is a technique used to track the passage of an isotope, or an atom with a variation, through a reaction, metabolic pathway, or cell. The reactant is 'labeled' by replacing specific atoms by their isotope. The reactant is then allowed to undergo the reaction. The position of the isotopes in the products is measured to determine the sequence the isotopic atom followed in the reaction or the cell's metabolic pathway. The nuclides used in isotopic labeling may be stable nuclides or radionuclides. In the latter case, the labeling is called radiolabeling.In isotopic labeling, there are multiple ways to detect the presence of labeling isotopes; through their mass, vibrational mode, or radioactive decay. Mass spectrometry detects the difference in an isotope's mass, while infrared spectroscopy detects the difference in the isotope's vibrational modes. Nuclear magnetic resonance detects atoms with different gyromagnetic ratios. The radioactive decay can be detected through an ionization chamber or autoradiographs of gels.An example of the use of isotopic labeling is the study of phenol (C6H5OH) in water by replacing common hydrogen (protium) with deuterium (deuterium labeling). Upon adding phenol to deuterated water (water containing D2O in addition to the usual H2O), the substitution of deuterium for the hydrogen is observed in phenol's hydroxyl group (resulting in C6H5OD), indicating that phenol readily undergoes hydrogen-exchange reactions with water. Only the hydroxyl group was affected, indicating that the other 5 hydrogen atoms did not participate in these exchange reactions.