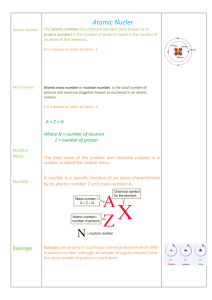
Chemistry in Biology
... A catalyst is a substance that lowers the activation energy needed to start a chemical reaction. It does not increase how much product is made and it does not get used up in the reaction. Enzymes are biological catalysts. ...
... A catalyst is a substance that lowers the activation energy needed to start a chemical reaction. It does not increase how much product is made and it does not get used up in the reaction. Enzymes are biological catalysts. ...
HIBBING COMMUNITY COLLEGE
... 46. explain the concept of thermal equilibrium and the chemistry of fire. 47. assign oxidation numbers and identify oxidation and reduction processes. 48. describe endothermic and exothermic processes. 49. describe what happens when heat is added to a substance. 50. calculate the heat needed for pha ...
... 46. explain the concept of thermal equilibrium and the chemistry of fire. 47. assign oxidation numbers and identify oxidation and reduction processes. 48. describe endothermic and exothermic processes. 49. describe what happens when heat is added to a substance. 50. calculate the heat needed for pha ...
Name ______ Period ______ 7th Grade Science Study Guide 1 7
... b. Qualitative measurements use numbers/senses. 8. Identify the following as QN for Quantitative and QL for qualitiative. _______ The air smells like smoke. ...
... b. Qualitative measurements use numbers/senses. 8. Identify the following as QN for Quantitative and QL for qualitiative. _______ The air smells like smoke. ...
Exam 1 Review Sheet Honors Biology This is to be used for
... 41. If I give you a substance, you should be able to characterize it in terms of whether it is a molecule, element and/or compound. There are examples in the PowerPoint for you to try. 42. When does molecular motion stop (matter has zero kinetic energy)? How fast are atoms moving on average at room ...
... 41. If I give you a substance, you should be able to characterize it in terms of whether it is a molecule, element and/or compound. There are examples in the PowerPoint for you to try. 42. When does molecular motion stop (matter has zero kinetic energy)? How fast are atoms moving on average at room ...
Chapter 2 Notes
... Wednesday: Students watched video, “Chemistry of Caves” and filled out a classifying graphic organizer. EQ: What are chemical compounds? HWK: Read Chapter 2 Section 1 – Record vocabulary terms and answer section questions Thursday: Reviewed Chapter 2 Section 1 The Nature of Matter Outline. When an a ...
... Wednesday: Students watched video, “Chemistry of Caves” and filled out a classifying graphic organizer. EQ: What are chemical compounds? HWK: Read Chapter 2 Section 1 – Record vocabulary terms and answer section questions Thursday: Reviewed Chapter 2 Section 1 The Nature of Matter Outline. When an a ...
AP Chemistry Review Assignment Brown and LeMay: Chemistry the
... The last part of this section, including how to determine the formula of a hydrate, and how to use combustion analyses to determine empirical formulas will be addressed early in the semester, probably Thurs., Aug. 20. 43. Give the empirical formula of each of the following compounds if a sample cont ...
... The last part of this section, including how to determine the formula of a hydrate, and how to use combustion analyses to determine empirical formulas will be addressed early in the semester, probably Thurs., Aug. 20. 43. Give the empirical formula of each of the following compounds if a sample cont ...
Chapter 2 power point
... Filtration: Separates components of a mixture based upon differences in particle size. Filtration usually involves separating a precipitate from solution. Crystallization: Separation is based upon differences in solubility of the components in a mixture. Distillation: Separation is based upon differ ...
... Filtration: Separates components of a mixture based upon differences in particle size. Filtration usually involves separating a precipitate from solution. Crystallization: Separation is based upon differences in solubility of the components in a mixture. Distillation: Separation is based upon differ ...
Atomic Nuclei - RAJEEV Classes
... number increases by 1 but mass number remains same. c)The emission of a γ-particle does not change the mass number or the atomic number of the radioactive nucleus. The γ-particle emission by a radioactive nucleus lowers its energy state. ...
... number increases by 1 but mass number remains same. c)The emission of a γ-particle does not change the mass number or the atomic number of the radioactive nucleus. The γ-particle emission by a radioactive nucleus lowers its energy state. ...
Chapter 2 Lecture notes
... 1. All matter is composed of atoms. The atom is the smallest body that retains the unique identity of the element. 2. Atoms of one element cannot be converted into atoms of another element in a chemical reaction. Elements can only be converted into other elements in nuclear reactions. 3. All atoms o ...
... 1. All matter is composed of atoms. The atom is the smallest body that retains the unique identity of the element. 2. Atoms of one element cannot be converted into atoms of another element in a chemical reaction. Elements can only be converted into other elements in nuclear reactions. 3. All atoms o ...
CHEM 115 EXAM #1 - chem.wilkes.edu
... temperature is listed as 320 K. Express this temperature in both ºC and ºF. °C = K – 273.15 = 46.85 (rounds to 47°C) °F = 1.8(°C) +32 = 116°F (note, I used 46.85 in this second calculation and then rounded) ...
... temperature is listed as 320 K. Express this temperature in both ºC and ºF. °C = K – 273.15 = 46.85 (rounds to 47°C) °F = 1.8(°C) +32 = 116°F (note, I used 46.85 in this second calculation and then rounded) ...
Determination of Equilibrium Constants using NMR Spectroscopy
... A photon whose frequency is such that its energy matches the energy difference between the spin states can be absorbed: ...
... A photon whose frequency is such that its energy matches the energy difference between the spin states can be absorbed: ...
4 ATOMIC STRUCTURE NOTES __ /__ pts
... Part B True-False Classify each of these statements as always true, AT; sometimes true, ST; or never true, NT. ________ 11. The atomic number of an element is the sum of the protons and electrons in an atom of that element. ________ 12. The atomic number of an atom is the total number of protons in ...
... Part B True-False Classify each of these statements as always true, AT; sometimes true, ST; or never true, NT. ________ 11. The atomic number of an element is the sum of the protons and electrons in an atom of that element. ________ 12. The atomic number of an atom is the total number of protons in ...
AP Chemistry Summer Assignment Summer 2015 Ms. Osquist
... • Determine if an element is a metal or nonmetal and the charge of its most common ion (for A-group elements) using the periodic table. • Write the formula of an ionic compound given its name (including polyatomic ions). • Explain how a cation or anion would form from a neutral atom. • Identify the ...
... • Determine if an element is a metal or nonmetal and the charge of its most common ion (for A-group elements) using the periodic table. • Write the formula of an ionic compound given its name (including polyatomic ions). • Explain how a cation or anion would form from a neutral atom. • Identify the ...
Organic Compounds
... Lipids are organic molecules composed of Carbon, Hydrogen and Oxygen with the ratio Hydrogen atoms to Oxygen much higher than in carbohydrates, (this is why lipids contains more energy than carbohydrates). The most important molecules of lipids are the fats. Fats are composed of a glycerol molecule ...
... Lipids are organic molecules composed of Carbon, Hydrogen and Oxygen with the ratio Hydrogen atoms to Oxygen much higher than in carbohydrates, (this is why lipids contains more energy than carbohydrates). The most important molecules of lipids are the fats. Fats are composed of a glycerol molecule ...
Chemistry Atoms Learning Objectives Atoms Essential knowledge
... in the order of 10-15 m. We now know that they are not the fundamental particles. Take, for example, a proton. It is now believed a proton is made of three fundamental particles called quarks. There are six different types of quarks, of which only up (u) quark and down (d) quark made up of protons: ...
... in the order of 10-15 m. We now know that they are not the fundamental particles. Take, for example, a proton. It is now believed a proton is made of three fundamental particles called quarks. There are six different types of quarks, of which only up (u) quark and down (d) quark made up of protons: ...
24.8 Fates of the Carbon Atoms from Amino Acids
... 24.8 Fates of the Carbon Atoms from Amino Acids Carbon atoms from degraded amino acids are converted to the intermediates of the citric acid cycle or other pathways. Learning Goal Describe where carbon atoms from amino acids enter the citric acid cycle or other pathways. General, Organic, and Biolog ...
... 24.8 Fates of the Carbon Atoms from Amino Acids Carbon atoms from degraded amino acids are converted to the intermediates of the citric acid cycle or other pathways. Learning Goal Describe where carbon atoms from amino acids enter the citric acid cycle or other pathways. General, Organic, and Biolog ...
chapter 4
... but different than A and BA element Atoms of element A and B can be can be physically chemically combined mixed together as a compound ...
... but different than A and BA element Atoms of element A and B can be can be physically chemically combined mixed together as a compound ...
Chapter 2: Atoms, Molecules, and Ions
... Acid Nomenclature Compound which can release H+ in water are called Acids. (aqueous, aq.,- means dissolved in water) Nomenclature is related to that of Ionic Compounds -If salt is binary, it forms binary acids : ...
... Acid Nomenclature Compound which can release H+ in water are called Acids. (aqueous, aq.,- means dissolved in water) Nomenclature is related to that of Ionic Compounds -If salt is binary, it forms binary acids : ...
8F Compounds and Mixtures
... reaction, i.e. any process in which atoms become joined in different ways. The steps for writing a word equation are: ...
... reaction, i.e. any process in which atoms become joined in different ways. The steps for writing a word equation are: ...
Writing and Balancing Chemical Equations
... 1) Assemble the correct formulas for all the reactants and products, using “+” and “→” 2) Count the number of atoms of each type appearing on both sides 3) Balance the elements one at a time by adding coefficients (the numbers in front) where you need more - save balancing the H and O until LAST! ...
... 1) Assemble the correct formulas for all the reactants and products, using “+” and “→” 2) Count the number of atoms of each type appearing on both sides 3) Balance the elements one at a time by adding coefficients (the numbers in front) where you need more - save balancing the H and O until LAST! ...
Organic Molecules and Water 1. In most animal cells, a complex
... molecules. The backbones of carbon molecules can be of any size and may contain from one carbon atom to thousands of carbon atoms. 13. A fat is a kind of lipid that can store energy for a long period of time. Fats are made up of long chains of carbon and oxygen atoms bonded to a backbone structure. ...
... molecules. The backbones of carbon molecules can be of any size and may contain from one carbon atom to thousands of carbon atoms. 13. A fat is a kind of lipid that can store energy for a long period of time. Fats are made up of long chains of carbon and oxygen atoms bonded to a backbone structure. ...
Photosynthesis
... -- Rubisco has a ‘choice’ C4 Isotope Discrimination (less) -- Stomata more closed, internal CO2 concentrations lower -- PEP carboxylase has very high affinity for CO2 ...
... -- Rubisco has a ‘choice’ C4 Isotope Discrimination (less) -- Stomata more closed, internal CO2 concentrations lower -- PEP carboxylase has very high affinity for CO2 ...
Document
... 2. Atoms of one element cannot be converted into atoms of another element in a chemical reaction. Elements can only be converted into other elements in nuclear reactions. 3. All atoms of an element have the same number of protons and electrons, which determines the chemical behavior of the element. ...
... 2. Atoms of one element cannot be converted into atoms of another element in a chemical reaction. Elements can only be converted into other elements in nuclear reactions. 3. All atoms of an element have the same number of protons and electrons, which determines the chemical behavior of the element. ...
Chapter 4 powerpoint
... terms of relative charge and mass. • Describe the structure of the atom, including the locations of the subatomic particles. ...
... terms of relative charge and mass. • Describe the structure of the atom, including the locations of the subatomic particles. ...
Answers - U of L Class Index
... Lithium-6 and lithium-7 are the only two stable isotopes of lithium. [4 marks] Predict the likely modes of decay for isotopes of lithium that are heavier than the stable isotopes. Briefly explain your reasoning. β– decay [2 marks] Heavier isotopes of an element have more neutrons than the stable iso ...
... Lithium-6 and lithium-7 are the only two stable isotopes of lithium. [4 marks] Predict the likely modes of decay for isotopes of lithium that are heavier than the stable isotopes. Briefly explain your reasoning. β– decay [2 marks] Heavier isotopes of an element have more neutrons than the stable iso ...
Isotopic labeling

Isotopic labeling (or isotopic labelling) is a technique used to track the passage of an isotope, or an atom with a variation, through a reaction, metabolic pathway, or cell. The reactant is 'labeled' by replacing specific atoms by their isotope. The reactant is then allowed to undergo the reaction. The position of the isotopes in the products is measured to determine the sequence the isotopic atom followed in the reaction or the cell's metabolic pathway. The nuclides used in isotopic labeling may be stable nuclides or radionuclides. In the latter case, the labeling is called radiolabeling.In isotopic labeling, there are multiple ways to detect the presence of labeling isotopes; through their mass, vibrational mode, or radioactive decay. Mass spectrometry detects the difference in an isotope's mass, while infrared spectroscopy detects the difference in the isotope's vibrational modes. Nuclear magnetic resonance detects atoms with different gyromagnetic ratios. The radioactive decay can be detected through an ionization chamber or autoradiographs of gels.An example of the use of isotopic labeling is the study of phenol (C6H5OH) in water by replacing common hydrogen (protium) with deuterium (deuterium labeling). Upon adding phenol to deuterated water (water containing D2O in addition to the usual H2O), the substitution of deuterium for the hydrogen is observed in phenol's hydroxyl group (resulting in C6H5OD), indicating that phenol readily undergoes hydrogen-exchange reactions with water. Only the hydroxyl group was affected, indicating that the other 5 hydrogen atoms did not participate in these exchange reactions.