Study guide for Midterm 3.
... c. In Chapter 17 we encountered an acyl group shuttle in the transfer of fatty acyl–CoA from the cytosol to the mitochondrion in preparation for β oxidation (see Fig. 17-6). One result of that shuttle was separation of the mitochondrial and cytosolic pools of CoA. Does the acetyl group shuttle also ...
... c. In Chapter 17 we encountered an acyl group shuttle in the transfer of fatty acyl–CoA from the cytosol to the mitochondrion in preparation for β oxidation (see Fig. 17-6). One result of that shuttle was separation of the mitochondrial and cytosolic pools of CoA. Does the acetyl group shuttle also ...
AP Chem Summer Assign Gen Chem Rev Problems
... b. Due to heat loss to the surroundings, the amount you calculated is lower than it should have been. Suppose under perfect conditions the heat transferred should have been 943 cal. Calculate the Percent Error of the experiment in (a). c. How many joules of energy are required to melt a 17 gram ice ...
... b. Due to heat loss to the surroundings, the amount you calculated is lower than it should have been. Suppose under perfect conditions the heat transferred should have been 943 cal. Calculate the Percent Error of the experiment in (a). c. How many joules of energy are required to melt a 17 gram ice ...
Lesson Overview
... Covalent bonds called peptide bonds link amino acids together to form a polypeptide. A protein is a functional molecule built from one or more polypeptides. ...
... Covalent bonds called peptide bonds link amino acids together to form a polypeptide. A protein is a functional molecule built from one or more polypeptides. ...
NSCC Chem 121 chapter2
... • An example of an isotope symbol is 28 Ni. This symbol represents an isotope of nickel that contains 28 protons and 32 neutrons in the nucleus. • Isotopes are also represented by the notation: Name-A, where Name is the name of the element and A is the mass number of the isotope. • An example of thi ...
... • An example of an isotope symbol is 28 Ni. This symbol represents an isotope of nickel that contains 28 protons and 32 neutrons in the nucleus. • Isotopes are also represented by the notation: Name-A, where Name is the name of the element and A is the mass number of the isotope. • An example of thi ...
QUIZ: History of Atomic Structure
... D) Each atom of an element is identical to every other atom of that element. E) All matter is composed of atoms. 6. Rutherford's experiment was important because it showed that: A) radioactive elements give off alpha particles B) gold foil can be made only a few atoms thick C) a zinc sulfide screen ...
... D) Each atom of an element is identical to every other atom of that element. E) All matter is composed of atoms. 6. Rutherford's experiment was important because it showed that: A) radioactive elements give off alpha particles B) gold foil can be made only a few atoms thick C) a zinc sulfide screen ...
Atomic Structure - Hudson City School District
... Why do atoms bond? • To have filled outer electron shells! ...
... Why do atoms bond? • To have filled outer electron shells! ...
Class 9 CBSE Test paper Solved Chapter 3: Atoms and...
... Ans: Atomic number is the total number of protons present in the nucleus of an atom. Mass number is the sum of protons and neutrons present in the nucleus of an atom. Atomic number is the fundamental attribute of elements because all elements are characterised by their atomic numbers. ...
... Ans: Atomic number is the total number of protons present in the nucleus of an atom. Mass number is the sum of protons and neutrons present in the nucleus of an atom. Atomic number is the fundamental attribute of elements because all elements are characterised by their atomic numbers. ...
Students will review concepts from their quiz and then correct it at
... A pure substance containing two or more kinds of __atoms__. The atoms are ___chemically___ combined in some way. Often times (but not always) they come together to form groups of atoms called molecules. A compound is always homogeneous (uniform). Compounds ___cannot___ be separated by physical means ...
... A pure substance containing two or more kinds of __atoms__. The atoms are ___chemically___ combined in some way. Often times (but not always) they come together to form groups of atoms called molecules. A compound is always homogeneous (uniform). Compounds ___cannot___ be separated by physical means ...
File
... The Structure of the Atom An atom is the smallest particle of an element that retains the chemical properties of that element. All atoms are composed of two regions: – Nucleus: very small dense region located in the center of the atom. »Made up of at least one positive particle, proton, and usually ...
... The Structure of the Atom An atom is the smallest particle of an element that retains the chemical properties of that element. All atoms are composed of two regions: – Nucleus: very small dense region located in the center of the atom. »Made up of at least one positive particle, proton, and usually ...
Key - Seattle Central College
... law of definite proportions (also the law of constant composition): – A compound always has same elements in the same proportion by mass – i.e., a compound always has the same formula → Water is always H2O. law of multiple proportions: – Two or more elements can combine to form different compounds – ...
... law of definite proportions (also the law of constant composition): – A compound always has same elements in the same proportion by mass – i.e., a compound always has the same formula → Water is always H2O. law of multiple proportions: – Two or more elements can combine to form different compounds – ...
Carbon Compounds
... believing they were different from compounds in nonliving things. Today, organic chemistry means the study of compounds that contain bonds between carbon atoms, while inorganic chemistry is the study of all other compounds. ...
... believing they were different from compounds in nonliving things. Today, organic chemistry means the study of compounds that contain bonds between carbon atoms, while inorganic chemistry is the study of all other compounds. ...
Atoms, Elements, and Ions
... • The quantity and mass of reactants equals the quantity and mass of the products. ...
... • The quantity and mass of reactants equals the quantity and mass of the products. ...
5 Early Atomic Theory and Structure Chapter Outline Early Theories
... Protons and neutrons are located in the nucleus. Electrons are dispersed throughout the remainder of the atom (mainly open space). Neutral atoms contain the same number of protons and neutrons to maintain charge balance. © 2014 John Wiley & Sons, Inc. All rights reserved. ...
... Protons and neutrons are located in the nucleus. Electrons are dispersed throughout the remainder of the atom (mainly open space). Neutral atoms contain the same number of protons and neutrons to maintain charge balance. © 2014 John Wiley & Sons, Inc. All rights reserved. ...
Review Session 3 Problems
... 1) Titanium tetrachloride is an important industrial chemical. It is used for preparing the TiO2, the white pigment in paints and paper. It can be made using an impure titanium ore(often impure TiO2) with carbon and chlorine. ...
... 1) Titanium tetrachloride is an important industrial chemical. It is used for preparing the TiO2, the white pigment in paints and paper. It can be made using an impure titanium ore(often impure TiO2) with carbon and chlorine. ...
Chapter 3 Test Review
... - Homework for all of chapter one is due before break of else all days will be minimal. ...
... - Homework for all of chapter one is due before break of else all days will be minimal. ...
Chapter 5
... Unless you are TOLD that the atom has a charge, you should assume it has no charge, and therefore, # of protons = # of electrons. The number of protons cannot change. If the number of protons changes, it’s no longer the same element. Atoms can gain or lose electrons, but they can NOT gain or los ...
... Unless you are TOLD that the atom has a charge, you should assume it has no charge, and therefore, # of protons = # of electrons. The number of protons cannot change. If the number of protons changes, it’s no longer the same element. Atoms can gain or lose electrons, but they can NOT gain or los ...
Isotopes are atoms of the same element that have different masses
... 7. An isotope of xenon has an atomic number of 54 and contains 77 neutrons. What is the mass of this isotope? 8. What is the mass number of uranium-234? 9. How many neutrons are in uranium 234? 10. Silicon is very important to the semiconductor industry. The three naturally occurring isotopes of sil ...
... 7. An isotope of xenon has an atomic number of 54 and contains 77 neutrons. What is the mass of this isotope? 8. What is the mass number of uranium-234? 9. How many neutrons are in uranium 234? 10. Silicon is very important to the semiconductor industry. The three naturally occurring isotopes of sil ...
Chapter 2
... Mass number = mass of the protons and neutrons Mass numbers of atoms of an element are not all identical Isotopes are structural variations of elements that differ in the number of neutrons they contain ...
... Mass number = mass of the protons and neutrons Mass numbers of atoms of an element are not all identical Isotopes are structural variations of elements that differ in the number of neutrons they contain ...
File
... with electrons arranged around it. Protons and neutrons have a relative mass unit of 1 Electrons have a very small mass compared to protons and neutrons ...
... with electrons arranged around it. Protons and neutrons have a relative mass unit of 1 Electrons have a very small mass compared to protons and neutrons ...
printer-friendly sample test questions
... Identify the number of iron and oxygen atoms in the reactants that will balance the equation. A. Four iron atoms and four oxygen atoms. B. Four iron atoms and six oxygen atoms. C. Two iron atoms and two oxygen atoms. D. Two iron atoms and three oxygen atoms. 3rd Item Specification: Explain that the ...
... Identify the number of iron and oxygen atoms in the reactants that will balance the equation. A. Four iron atoms and four oxygen atoms. B. Four iron atoms and six oxygen atoms. C. Two iron atoms and two oxygen atoms. D. Two iron atoms and three oxygen atoms. 3rd Item Specification: Explain that the ...
Biology * Introduction to Organic Chemistry
... most other biological molecules, lipids are hydrophobic (waterfearing). You can see this chemical behavior in an unshaken bottle of salad dressing. The oil (a type of lipid) separates from the vinegar (which is mostly water). ...
... most other biological molecules, lipids are hydrophobic (waterfearing). You can see this chemical behavior in an unshaken bottle of salad dressing. The oil (a type of lipid) separates from the vinegar (which is mostly water). ...
IT IS ELEMENTARY - the OLLI at UCI Blog
... and animal origin • These elements or their very simple compounds can kill—most commonly by interfering with cellular access to oxygen • Nitrogen N2 • Carbon dioxide CO2 • Carbon monoxide CO • Hydrogen cyanide HCN ...
... and animal origin • These elements or their very simple compounds can kill—most commonly by interfering with cellular access to oxygen • Nitrogen N2 • Carbon dioxide CO2 • Carbon monoxide CO • Hydrogen cyanide HCN ...
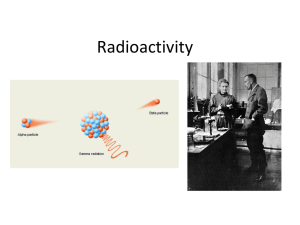
Radioactivity
... • The discovery of radioactivity by Becquerel and the Curies showed that one of Dalton’s ideas, that matter is indestructible and indivisible, is not always true. • Certain isotopes, because of their size and/or ratio of protons and neutrons are not stable. • Radioisotopes have unstable, high energy ...
... • The discovery of radioactivity by Becquerel and the Curies showed that one of Dalton’s ideas, that matter is indestructible and indivisible, is not always true. • Certain isotopes, because of their size and/or ratio of protons and neutrons are not stable. • Radioisotopes have unstable, high energy ...
46 Pd Palladium 106.4
... ____ 4. What did Democritus, Dalton, Thomson, Rutherford, and Bohr all have in common? A. They each identified new elements. B. They each identified new isotopes of atoms. C. They each contributed to the development of the atomic theory. D. They each conducted experiments in which particles collided ...
... ____ 4. What did Democritus, Dalton, Thomson, Rutherford, and Bohr all have in common? A. They each identified new elements. B. They each identified new isotopes of atoms. C. They each contributed to the development of the atomic theory. D. They each conducted experiments in which particles collided ...
Isotopic labeling

Isotopic labeling (or isotopic labelling) is a technique used to track the passage of an isotope, or an atom with a variation, through a reaction, metabolic pathway, or cell. The reactant is 'labeled' by replacing specific atoms by their isotope. The reactant is then allowed to undergo the reaction. The position of the isotopes in the products is measured to determine the sequence the isotopic atom followed in the reaction or the cell's metabolic pathway. The nuclides used in isotopic labeling may be stable nuclides or radionuclides. In the latter case, the labeling is called radiolabeling.In isotopic labeling, there are multiple ways to detect the presence of labeling isotopes; through their mass, vibrational mode, or radioactive decay. Mass spectrometry detects the difference in an isotope's mass, while infrared spectroscopy detects the difference in the isotope's vibrational modes. Nuclear magnetic resonance detects atoms with different gyromagnetic ratios. The radioactive decay can be detected through an ionization chamber or autoradiographs of gels.An example of the use of isotopic labeling is the study of phenol (C6H5OH) in water by replacing common hydrogen (protium) with deuterium (deuterium labeling). Upon adding phenol to deuterated water (water containing D2O in addition to the usual H2O), the substitution of deuterium for the hydrogen is observed in phenol's hydroxyl group (resulting in C6H5OD), indicating that phenol readily undergoes hydrogen-exchange reactions with water. Only the hydroxyl group was affected, indicating that the other 5 hydrogen atoms did not participate in these exchange reactions.