1 - contentextra
... Anthocyanins The anthocyanins are the most widely distributed pigment in plants and are present, for example, in strawberries, plums, cranberries, blueberries and raspberries. They are a sub-class of flavonoids responsible for a range of colours in fruits and vegetables including yellow, red and blu ...
... Anthocyanins The anthocyanins are the most widely distributed pigment in plants and are present, for example, in strawberries, plums, cranberries, blueberries and raspberries. They are a sub-class of flavonoids responsible for a range of colours in fruits and vegetables including yellow, red and blu ...
radicals
... Decarboxylation produce CO2, and are thus thermodynamically favorable. These reactions proceed most readily when the COOH moiety is attached to an electronegative atom, like oxygen or nitrogen. ...
... Decarboxylation produce CO2, and are thus thermodynamically favorable. These reactions proceed most readily when the COOH moiety is attached to an electronegative atom, like oxygen or nitrogen. ...
You Light Up My Life
... Structural isomers – differ in the arrangement of their atoms Geometric isomers – differ in the arrangement of atoms around a double bond Enantiomers – Chiral Molecules – ...
... Structural isomers – differ in the arrangement of their atoms Geometric isomers – differ in the arrangement of atoms around a double bond Enantiomers – Chiral Molecules – ...
Reactions of Alkenes Organic Chemistry
... If an alcohol has its O—H bond converted to S—H, the alcohol is said to be a thiol. ...
... If an alcohol has its O—H bond converted to S—H, the alcohol is said to be a thiol. ...
Chapter 9 – Compounds of Carbon
... • The systemic name usually contains a prefix, a stem and a suffix. • The name of the hydrocarbon can be determined as follows: 1. Identify the longest chain of carbon atoms. The carbon atoms in this chain are numbered. 2. Check bonding, ane for single bonds. 3. Identify the side chain and the numbe ...
... • The systemic name usually contains a prefix, a stem and a suffix. • The name of the hydrocarbon can be determined as follows: 1. Identify the longest chain of carbon atoms. The carbon atoms in this chain are numbered. 2. Check bonding, ane for single bonds. 3. Identify the side chain and the numbe ...
FUNCTIONAL GROUPS
... • C-N, N-H, C=O bonds are polar, so molecules are usually polar • Primary and secondary amides experience hydrogen bonding • Soluble in water and other polar solvents, solubility decreases as the number of carbons increases • Primary amides have higher melting and boiling points than analogous ...
... • C-N, N-H, C=O bonds are polar, so molecules are usually polar • Primary and secondary amides experience hydrogen bonding • Soluble in water and other polar solvents, solubility decreases as the number of carbons increases • Primary amides have higher melting and boiling points than analogous ...
Chapter 7 Periodic Properties of the Elements
... 2. Sulfur (sulfur trioxide, SO3) 3. Sulfur (sulfur dioxide, SO2) 4. Carbon (carbon dioxide, CO2) ...
... 2. Sulfur (sulfur trioxide, SO3) 3. Sulfur (sulfur dioxide, SO2) 4. Carbon (carbon dioxide, CO2) ...
Chapters 6, 8
... 2HNO3(aq) + CuO(s) → Cu(NO3)2(aq) + H2O(l) 2. Formation of insoluble precipitate AgNO3(aq) + NaCl(aq) → AgCl(s) + NaNO3(aq) 3. Formation of a gas Heat in Chemical ...
... 2HNO3(aq) + CuO(s) → Cu(NO3)2(aq) + H2O(l) 2. Formation of insoluble precipitate AgNO3(aq) + NaCl(aq) → AgCl(s) + NaNO3(aq) 3. Formation of a gas Heat in Chemical ...
Chapter23
... Carboxylic Acids are abundant and widely distributed in nature. The low mass carboxylic acids are colorless volatile liquids with sharp, unpleasant odors. High mass carboxylic acids are waxy, oderless solids with low melting points. The table above lists the simplest organic acids. Low mass carboxyl ...
... Carboxylic Acids are abundant and widely distributed in nature. The low mass carboxylic acids are colorless volatile liquids with sharp, unpleasant odors. High mass carboxylic acids are waxy, oderless solids with low melting points. The table above lists the simplest organic acids. Low mass carboxyl ...
Technical Data Sheet - Lučební závody Kolín
... Synhydrid is a very effective hydridic reduction agent. It plays important role in organic chemistry and is mainly used as a versatile reduction agent for functional groups in organic molecules. Especially it is very effective for reduction of compounds containing carbonyl and carboxyl groups such a ...
... Synhydrid is a very effective hydridic reduction agent. It plays important role in organic chemistry and is mainly used as a versatile reduction agent for functional groups in organic molecules. Especially it is very effective for reduction of compounds containing carbonyl and carboxyl groups such a ...
Chapter 12
... charged electrons Also known as the "plum pudding" model of the atom Rutherford model of the atom (1910) Most of the mass of the atom, and all its positive charge, reside in a very small dense centrally located region called the "nucleus" Most of the total volume of the atom is empty space wit ...
... charged electrons Also known as the "plum pudding" model of the atom Rutherford model of the atom (1910) Most of the mass of the atom, and all its positive charge, reside in a very small dense centrally located region called the "nucleus" Most of the total volume of the atom is empty space wit ...
Contents CONCEPT Introduction to Structure of Atom Dalton`s
... Physical Properties of aldehydes and ketones Chemical Properties of Aldehydes and Ketones (nucleophilic addition reactions, nucleophilic additionelimination reactions, reduction, oxidation, Aldol condensation, Cannizzarro reaction, electrophiclic substitution in aromatic aldehydes) Structure of carb ...
... Physical Properties of aldehydes and ketones Chemical Properties of Aldehydes and Ketones (nucleophilic addition reactions, nucleophilic additionelimination reactions, reduction, oxidation, Aldol condensation, Cannizzarro reaction, electrophiclic substitution in aromatic aldehydes) Structure of carb ...
Introduction (HL)
... Enantiomers differ in only one aspect of their physical properties: they rotate the plane of plane-polarized light in opposite directions: - laevorotatory enantiomers counterclockwise (to the left) - dextrorotatory clockwise (to the right) ...
... Enantiomers differ in only one aspect of their physical properties: they rotate the plane of plane-polarized light in opposite directions: - laevorotatory enantiomers counterclockwise (to the left) - dextrorotatory clockwise (to the right) ...
ALKALI EARTH METALS Introduction Properties Beryllium
... The melting point, boiling point and the hardness of the elements decrease top to bottom. Beryllium is the hardest alkaline earth metal Barium is the softest alkaline earth metal. ...
... The melting point, boiling point and the hardness of the elements decrease top to bottom. Beryllium is the hardest alkaline earth metal Barium is the softest alkaline earth metal. ...
Chapter 4
... • This molecule has an OH (called a hydroxy group) attached to its backbone. • Compounds containing an OH group are called alcohols. • The hydroxy group makes the properties of ethanol very different from the properties of ethane. • Ethanol has lone pairs and polar bonds that make it reactive. ...
... • This molecule has an OH (called a hydroxy group) attached to its backbone. • Compounds containing an OH group are called alcohols. • The hydroxy group makes the properties of ethanol very different from the properties of ethane. • Ethanol has lone pairs and polar bonds that make it reactive. ...
Organic and Carbon Chemistry
... Ex: C4H10 2. Structural Formula: Also gives structural information (Lewis structure). Allows us to distinguish structural isomers. – Structural isomers (aka constitutional isomers): Same molecular formula, different structural formula (arrangements of ...
... Ex: C4H10 2. Structural Formula: Also gives structural information (Lewis structure). Allows us to distinguish structural isomers. – Structural isomers (aka constitutional isomers): Same molecular formula, different structural formula (arrangements of ...
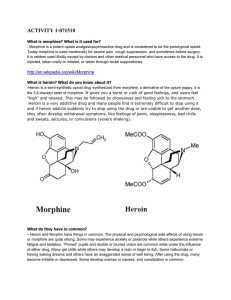
activity 1-071510 - ids
... What is heroin? What do you know about it? -Heroin is a semi-synthetic opioid drug synthesized from morphine, a derivative of the opium poppy. It is the 3,6-diacetyl ester of morphine. It gives you a burst or rush of good feelings, and users feel "high" and relaxed. This may be followed by drowsines ...
... What is heroin? What do you know about it? -Heroin is a semi-synthetic opioid drug synthesized from morphine, a derivative of the opium poppy. It is the 3,6-diacetyl ester of morphine. It gives you a burst or rush of good feelings, and users feel "high" and relaxed. This may be followed by drowsines ...
Test 2
... 12. Write a chemical equation for the reaction that occurs when aqueous solutions of calcium chloride and silver(I) nitrate are mixed, given the following information. ...
... 12. Write a chemical equation for the reaction that occurs when aqueous solutions of calcium chloride and silver(I) nitrate are mixed, given the following information. ...
Ch. 8 Notes (Chemical Reactions) Teacher Relearn
... Just use your ion sheet and find the names of the ions. cation name ...
... Just use your ion sheet and find the names of the ions. cation name ...
Chapter 4 Carbon
... Concept 4.1: Organic chemistry is the study of carbon compounds • Organic chemistry is the study of compounds that contain carbon • Organic compounds range from simple molecules to colossal ones • Most organic compounds contain hydrogen atoms in addition to carbon atoms ...
... Concept 4.1: Organic chemistry is the study of carbon compounds • Organic chemistry is the study of compounds that contain carbon • Organic compounds range from simple molecules to colossal ones • Most organic compounds contain hydrogen atoms in addition to carbon atoms ...
Organosulfur compounds

Organosulfur compounds are organic compounds that contain sulfur. They are often associated with foul odors, but many of the sweetest compounds known are organosulfur derivatives, e.g., saccharin. Nature abounds with organosulfur compounds—sulfur is essential for life. Of the 20 common amino acids, two (cysteine and methionine) are organosulfur compounds, and the antibiotics penicillin (pictured below) and sulfa drugs both contain sulfur. While sulfur-containing antibiotics save many lives, sulfur mustard is a deadly chemical warfare agent. Fossil fuels, coal, petroleum, and natural gas, which are derived from ancient organisms, necessarily contain organosulfur compounds, the removal of which is a major focus of oil refineries.Sulfur shares the chalcogen group with oxygen, selenium and tellurium, and it is expected that organosulfur compounds have similarities with carbon–oxygen, carbon–selenium and carbon–tellurium compounds, which is true to some extent.A classical chemical test for the detection of sulfur compounds is the Carius halogen method.