CH 3
... • Boiling Points: Compounds with hydrogen bonding have higher than expected boiling points: at SATP, C2H6 = gas but CH3OH = liquid. • Solubilities: Compounds with hydrogen bonds are usually soluble in water (a polar molecule): C6H14 = insoluble in water but C5H11OH = soluble in water. • Solubility d ...
... • Boiling Points: Compounds with hydrogen bonding have higher than expected boiling points: at SATP, C2H6 = gas but CH3OH = liquid. • Solubilities: Compounds with hydrogen bonds are usually soluble in water (a polar molecule): C6H14 = insoluble in water but C5H11OH = soluble in water. • Solubility d ...
CH 3
... • Boiling Points: Compounds with hydrogen bonding have higher than expected boiling points: at SATP, C2H6 = gas but CH3OH = liquid. • Solubilities: Compounds with hydrogen bonds are usually soluble in water (a polar molecule): C6H14 = insoluble in water but C5H11OH = soluble in water. • Solubility d ...
... • Boiling Points: Compounds with hydrogen bonding have higher than expected boiling points: at SATP, C2H6 = gas but CH3OH = liquid. • Solubilities: Compounds with hydrogen bonds are usually soluble in water (a polar molecule): C6H14 = insoluble in water but C5H11OH = soluble in water. • Solubility d ...
Alcohols/Wade: Reactions
... purified and reacted with magnesium and ether. Compound B is added to the resulting solution of the Grignard reagent. After hydrolysis, this solution is found to have 3,4dimethyl-3-hexanol. Propose the structures for A, B and C. PBr3 A ...
... purified and reacted with magnesium and ether. Compound B is added to the resulting solution of the Grignard reagent. After hydrolysis, this solution is found to have 3,4dimethyl-3-hexanol. Propose the structures for A, B and C. PBr3 A ...
Biology Fall 2013 Chapter 3 Biochemistry Study Guide
... Draw the atomic structure of the carbon atom (Bohr model, like we did in class) How many electrons are found in the outermost level for carbon? How many covalent bonds can carbon form with other atoms? Can carbon form bonds with other carbon atoms and with atoms of other elements? What is an organic ...
... Draw the atomic structure of the carbon atom (Bohr model, like we did in class) How many electrons are found in the outermost level for carbon? How many covalent bonds can carbon form with other atoms? Can carbon form bonds with other carbon atoms and with atoms of other elements? What is an organic ...
File
... a. If there are two or more longest chains of equal length, the one having the largest number of substituents is chosen. b. If both ends of the root chain have equidistant substituents: (i) Begin numbering at the end nearest a third substituent, if one is present. (ii) Begin numbering at the end nea ...
... a. If there are two or more longest chains of equal length, the one having the largest number of substituents is chosen. b. If both ends of the root chain have equidistant substituents: (i) Begin numbering at the end nearest a third substituent, if one is present. (ii) Begin numbering at the end nea ...
Honors Chemistry
... The additional notes on gases quiz will take place on ___________________________. It will be a 25 point quiz. It will have a 10 point matching section, and multiple short answer sections. You are required to know facts from the packet, equations, and be able to draw Lewis structures of the molecule ...
... The additional notes on gases quiz will take place on ___________________________. It will be a 25 point quiz. It will have a 10 point matching section, and multiple short answer sections. You are required to know facts from the packet, equations, and be able to draw Lewis structures of the molecule ...
course contents 160 - drseemaljelani
... science, and technology with a major in Chemistry. The Chemistry major enables you to view the world from a molecular perspective, and to solve complex problems that span the breadth of chemistry and other sciences. You can choose to specialize in Chemistry Through your studies you will gain transfe ...
... science, and technology with a major in Chemistry. The Chemistry major enables you to view the world from a molecular perspective, and to solve complex problems that span the breadth of chemistry and other sciences. You can choose to specialize in Chemistry Through your studies you will gain transfe ...
Chemistry 101 H Introduction to Organic Chemistry Chapter 6
... Before 19th Century, belief was: Compounds present in plants and animals required a “vital (life) force” for their creation. 1828, Friedrich Wohler carried out a laboratory synthesis of urea, a biological product formerly believed to be a product of the “vital force”. Wohler’s laboratory synthesis o ...
... Before 19th Century, belief was: Compounds present in plants and animals required a “vital (life) force” for their creation. 1828, Friedrich Wohler carried out a laboratory synthesis of urea, a biological product formerly believed to be a product of the “vital force”. Wohler’s laboratory synthesis o ...
Ch03_ Lecture
... • Vitamins are organic compounds needed in small amounts for normal cell function. • Most cannot be synthesized in our bodies, and must be obtained from the diet. • Most are identified by a letter, such as A, C, D, E, and K. • There are several different B vitamins, so a subscript is added to distin ...
... • Vitamins are organic compounds needed in small amounts for normal cell function. • Most cannot be synthesized in our bodies, and must be obtained from the diet. • Most are identified by a letter, such as A, C, D, E, and K. • There are several different B vitamins, so a subscript is added to distin ...
Electron Dot Diagrams for Four Simple Molecules ammonia, NH3
... (l) formaldehyde, CH2O (m) sulfur monoxide, SO (n) hydrogen cyanide, HCN (o) dioxygen difluoride, O2F2 (p) hypochlorous acid, HClO (q) isocyanic acid, NCOH (r) tetrafluoroethene, C2F4 (s) ethylamine, C2H7N (t) cyanogen, NCCN (or C2N2) (u) urea, CO(NH2)2 (v) acetonitrile, CH3CN ...
... (l) formaldehyde, CH2O (m) sulfur monoxide, SO (n) hydrogen cyanide, HCN (o) dioxygen difluoride, O2F2 (p) hypochlorous acid, HClO (q) isocyanic acid, NCOH (r) tetrafluoroethene, C2F4 (s) ethylamine, C2H7N (t) cyanogen, NCCN (or C2N2) (u) urea, CO(NH2)2 (v) acetonitrile, CH3CN ...
Organic Chemistry PowerPoint
... in a molecule, but gives no information on how they are arranged. Consider, for example, C4H10 Each isomer is a distinct compound, having unique physical and chemical properties. ...
... in a molecule, but gives no information on how they are arranged. Consider, for example, C4H10 Each isomer is a distinct compound, having unique physical and chemical properties. ...
Exam 1 - Winona State University
... III. Propose a synthetic sequence that could be used to carry out each of the following transformations in ...
... III. Propose a synthetic sequence that could be used to carry out each of the following transformations in ...
Unit 8 – Organic Chemistry
... • Up to this point, no organic compound had been synthesized from inorganic materials and, as a result, many scientists believed that organic compounds were formed only under the influence of a vital force. • It was Friedrich Wöhler (1800–1882) who, in 1828, made a remarkable discovery at the Univer ...
... • Up to this point, no organic compound had been synthesized from inorganic materials and, as a result, many scientists believed that organic compounds were formed only under the influence of a vital force. • It was Friedrich Wöhler (1800–1882) who, in 1828, made a remarkable discovery at the Univer ...
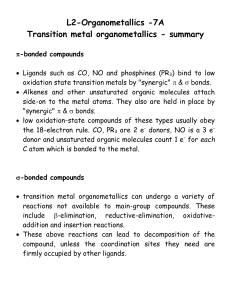
lect7
... the 18-electron rule. CO, PR3 are 2 e- donors, NO is a 3 edonor and unsaturated organic molecules count 1 e- for each C atom which is bonded to the metal. ...
... the 18-electron rule. CO, PR3 are 2 e- donors, NO is a 3 edonor and unsaturated organic molecules count 1 e- for each C atom which is bonded to the metal. ...
Physical Properties and Acidity of Carboxylic Acids
... acidity of carboxylic acids. However, inductive effects also play a role. For example, alcohols have pKa's of 16 or greater but their acidity is increased by electron withdrawing substituents on the alkyl group. The following diagram illustrates this factor for several simple inorganic and organic c ...
... acidity of carboxylic acids. However, inductive effects also play a role. For example, alcohols have pKa's of 16 or greater but their acidity is increased by electron withdrawing substituents on the alkyl group. The following diagram illustrates this factor for several simple inorganic and organic c ...
Chemistry of Carbon
... carbon compounds C atoms are versatile building blocks bonding properties 4 stable covalent bonds ...
... carbon compounds C atoms are versatile building blocks bonding properties 4 stable covalent bonds ...
Chem 3.5 Answers #7
... potassium dichromate solution. The aldehyde must be distilled off as it is made or it will oxidise further, up to the carboxylic acid. Ketones are made by the same oxidation reaction with secondary alcohols, but they do not need to be distilled of as the reaction proceeds. ...
... potassium dichromate solution. The aldehyde must be distilled off as it is made or it will oxidise further, up to the carboxylic acid. Ketones are made by the same oxidation reaction with secondary alcohols, but they do not need to be distilled of as the reaction proceeds. ...
Click on image to content
... an atom or a group of atoms bonded together in a unique fashion, which is usually the site of chemical reactivity in an organic molecule. For Example - The hydroxyl group in ethanol (C2H5–OH) is known as a functional group. The functional group in ethene is , and in ethyne, it is – C ≡ C – . Thu ...
... an atom or a group of atoms bonded together in a unique fashion, which is usually the site of chemical reactivity in an organic molecule. For Example - The hydroxyl group in ethanol (C2H5–OH) is known as a functional group. The functional group in ethene is , and in ethyne, it is – C ≡ C – . Thu ...
Slide 1 In this lesson, we will give you a general
... Let us look at the definition of organic molecules. Organic Molecules are the compounds containing carbon atoms. We do not include Carbon dioxide and diamonds under this category. Early Thoughts were that only living things could synthesize organic compounds. But, in 1800, an organic compound was sy ...
... Let us look at the definition of organic molecules. Organic Molecules are the compounds containing carbon atoms. We do not include Carbon dioxide and diamonds under this category. Early Thoughts were that only living things could synthesize organic compounds. But, in 1800, an organic compound was sy ...
CH 115 Exam 2 - UAB General Chemistry Supplemental Instruction
... 5. What is the theoretical yield of PbCl2 from the equation below given 14.8 g of KCl and 51.2 g of Pb(NO3)2? ...
... 5. What is the theoretical yield of PbCl2 from the equation below given 14.8 g of KCl and 51.2 g of Pb(NO3)2? ...
04_lecture_presentation
... Concept 4.1: Organic chemistry is the study of carbon compounds • Organic chemistry is the study of compounds that contain carbon • Organic compounds range from simple molecules to colossal ones • Most organic compounds contain hydrogen atoms in addition to carbon atoms ...
... Concept 4.1: Organic chemistry is the study of carbon compounds • Organic chemistry is the study of compounds that contain carbon • Organic compounds range from simple molecules to colossal ones • Most organic compounds contain hydrogen atoms in addition to carbon atoms ...
Organosulfur compounds

Organosulfur compounds are organic compounds that contain sulfur. They are often associated with foul odors, but many of the sweetest compounds known are organosulfur derivatives, e.g., saccharin. Nature abounds with organosulfur compounds—sulfur is essential for life. Of the 20 common amino acids, two (cysteine and methionine) are organosulfur compounds, and the antibiotics penicillin (pictured below) and sulfa drugs both contain sulfur. While sulfur-containing antibiotics save many lives, sulfur mustard is a deadly chemical warfare agent. Fossil fuels, coal, petroleum, and natural gas, which are derived from ancient organisms, necessarily contain organosulfur compounds, the removal of which is a major focus of oil refineries.Sulfur shares the chalcogen group with oxygen, selenium and tellurium, and it is expected that organosulfur compounds have similarities with carbon–oxygen, carbon–selenium and carbon–tellurium compounds, which is true to some extent.A classical chemical test for the detection of sulfur compounds is the Carius halogen method.