* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download FUNCTIONAL GROUPS
Strychnine total synthesis wikipedia , lookup
Homoaromaticity wikipedia , lookup
Aromaticity wikipedia , lookup
Petasis reaction wikipedia , lookup
Organosulfur compounds wikipedia , lookup
Hydroformylation wikipedia , lookup
Asymmetric induction wikipedia , lookup
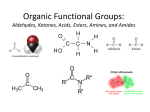
FUNCTIONAL GROUPS FUNCTIONAL GROUPS • A functional group is a cluster of atoms within a molecule that have specific reactivity patterns • Compounds with the same functional group have similar physical properties • The general formula for an organic compound with a functional group is R + functional group • R stands for the alkyl group (carbon chain) • If there is more than one alkyl group, we use R’, R’’ PHYSICAL PROPERTIES AND FORCES BETWEEN MOLECULES • Physical properties are largely dictated by intermolecular forces • There are three main types • Hydrogen bonding strong intermolecular attraction between • The H atom on a N-H, O-H, F-H group of one molecule and the N, O, or F of another molecule • Dipole-Dipole interactions: between polar molar molecules • Dispersion forces: between all covalent molecules • Very weak for small molecules, but they strengthen as the size of the molecule get larger SOME INTERESTING POINTS ABOUT INTERMOLECULAR FORCES SINGLE-BONDED FUNCTIONAL GROUPS 1. ALCOHOLS ALCOHOLS PROPERTIES OF ALCOHOLS 2. ORGANIC HALIDES PROPERTIES OF ORGANIC HALIDES 3. ETHERS 4. AMINES PROPERTIES OF AMINES PROPERTIES OF ETHERS FUNCTIONAL GROPS WITH THE C=O BOND THE CARBONYL GROUP • Found in many compounds • One of the most interesting functional groups O • The double bond makes it more reactive the a C-O single bond (in alcohols, ethers) • Different reactivity patterns CH2 1. ALDEHYDES AND KETONES • Hydrocarbons with a C=O functional group • If the carbonyl group is at the end of the parent chain, it is an O aldehyde C R H • If the carbonyl group is in the middle of the parent chain, it is an ketone R O O C C H R R Terminal carbonyl group gives an aldehyde Internal carbonyl group gives a ketone General formula: R-CHO General formula: R-CO-R’ Examples O H3C CH2 C CH2 CH2 H O H3C CH2 C CH2 CH2 CH2 CH2 CH3 • Pentanal • 3-octanone • Aldehydes end in “–al” • Ketones end in “–one” PROPERTIES OF ALDEHYDES AND KETONES • The C=O is polar, so aldehydes and ketones are usually polar • Hydrogen bonding cannot occur between molecules of these compounds • But, since oxygen has two lone pairs, it can form weak hydrogen bonds with water • The low molecule mass chains are soluble in polar solvents, as the number of carbons increase, the solubility decreases • Boiling points are lower than analogous alochols • i.e. Ethanol = 78.9°C; Ethanal = 20.2°C • Propanol = 97°C; Propanone (acetone) = 56°C • Odours: • Aldehydes: strong pungent • Ketones tend to smell sweet • They are good organic solvents for both polar and non-polar compounds • Acetone = propanone: one of the most common organic solvents in chemistry labs • Remember: like-dissolves-like • These are polar/non-polar at the same time, so they can dissolve both 2. CARBOXYLIC ACIDS • The carboxyl group characterizes organic acids (proton donors) • Recall: amines = organic bases • These are always terminal, since they take up three of carbons four bonds • General formula: R-COOH • Names always end in –oic acid • Example: pentanoic acid O C R OH PROPERTIES OF CARBOXYLIC ACIDS • Similar to alcohols • Hydrogen bonding occurs between chains and with water molecules • Melting and boiling points are higher than parent alkanes; increase with number of carboxyl groups • Have unpleasant odours • The H on the OH means that it can easily donate the H to another molecule 3. ESTERS • Derivatives of carboxylic acids, where the ‘H’ of COOH has been replaced with an alkyl group O • Combination of carboxylic acid and alcohols • Names in “-oate” R C R O 1 PROPERTIES OF ESTERS • Usually polar molecules • Do not have O-H bond, therefore do not form hydrogen bonds with each other • Due to lone pairs on O atoms, can accept a hydrogen bon from water • Low molecular masses are soluble in water and polar solvents • Low boiling points, usually volatile liquids (combustible/explosive) • Have pleasant odours and tastes • Used as perfumes and artificial flavours 4. AMIDES • Amides are derivatives of carboxylic acids, where the OH of the COOH has replaced with an amine group O • Combining a carboxylic acid and an amine • Can be primary amides, secondary or tertiary • Names end in: “amide” C R R N R 2 1 PROPERTIES OF AMIDES • C-N, N-H, C=O bonds are polar, so molecules are usually polar • Primary and secondary amides experience hydrogen bonding • Soluble in water and other polar solvents, solubility decreases as the number of carbons increases • Primary amides have higher melting and boiling points than analogous carboxylic acids • Solid at room temperature • Form the backbone of all proteins Homework • Practice Worksheet