1. Protein Interactions
... Size: Larger molecules have more active sites Structure: the stability (strength of intramolecular bonds) and molecule unfolding rate ...
... Size: Larger molecules have more active sites Structure: the stability (strength of intramolecular bonds) and molecule unfolding rate ...
PPT - LSU Physics & Astronomy
... A radio station transmits a 10 kW signal at a frequency of 100 MHz. At a distance of 1km from the antenna, find the amplitude of the electric and magnetic field strengths, and the energy incident normally on a square plate of side 10cm in 5 minutes. ...
... A radio station transmits a 10 kW signal at a frequency of 100 MHz. At a distance of 1km from the antenna, find the amplitude of the electric and magnetic field strengths, and the energy incident normally on a square plate of side 10cm in 5 minutes. ...
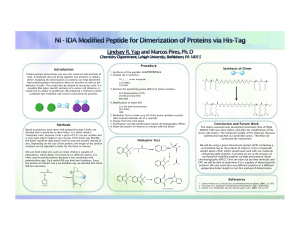
Introduction Methods Procedure Conclusion and Future Work
... 3. Remove the protecting group (Mmt) on lysine residues: ...
... 3. Remove the protecting group (Mmt) on lysine residues: ...
Let`s Get Pumped Up about Proteins!!!
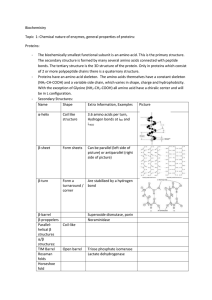
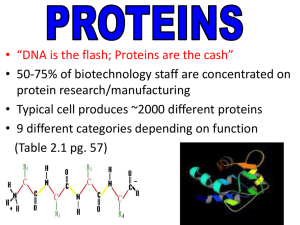
... • Typical cell produces ~2000 different proteins • 9 different categories depending on function (Table 2.1 pg. 57) ...
... • Typical cell produces ~2000 different proteins • 9 different categories depending on function (Table 2.1 pg. 57) ...
DN: Protein
... sequence of the 20 different amino acids as illustrated on the left. In the feed lab, protein is distinguishable from carbohydrate and lipid due to its content of nitrogen (N) feed proteins typically contain about 16% N. This property makes it possible to estimate the protein content of a feedstuff ...
... sequence of the 20 different amino acids as illustrated on the left. In the feed lab, protein is distinguishable from carbohydrate and lipid due to its content of nitrogen (N) feed proteins typically contain about 16% N. This property makes it possible to estimate the protein content of a feedstuff ...
Study Guide 2—Chemical Principles 1. Understand, define and be
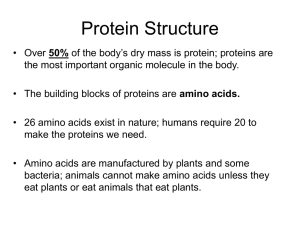
... monomer, polymer, macromolecule, carbohydrate, monosaccharide, disaccharide, polysaccharide, lipid, triglyceride, fatty acid, glycerol, phospholipid, sterol, steroid, protein, amino acid, peptide bond, primary structure, secondary structure, tertiary structure, quaternary structure, denature, nuclei ...
... monomer, polymer, macromolecule, carbohydrate, monosaccharide, disaccharide, polysaccharide, lipid, triglyceride, fatty acid, glycerol, phospholipid, sterol, steroid, protein, amino acid, peptide bond, primary structure, secondary structure, tertiary structure, quaternary structure, denature, nuclei ...
Research Interests
... and endonucleases which are controllable by light. Gram negative bacteria deploy various secretion systems for exporting proteins to eukaryotic hosts. We study the Type III secretion systems (T3SS) which are directly related to pathogenicity and essential mediators of the interactions between bacter ...
... and endonucleases which are controllable by light. Gram negative bacteria deploy various secretion systems for exporting proteins to eukaryotic hosts. We study the Type III secretion systems (T3SS) which are directly related to pathogenicity and essential mediators of the interactions between bacter ...
Honors Biology Name Biochemistry Exam Review #1 Period _____
... The material an enzyme works on is called the substrates. The pocket or groove where the substrate fits into on the enzyme is called the active site. (See diagram in enzyme notes for enzyme structure) Enzymes are named for the substrate that they work with. Names usually end in –ase (ex. Lactase, He ...
... The material an enzyme works on is called the substrates. The pocket or groove where the substrate fits into on the enzyme is called the active site. (See diagram in enzyme notes for enzyme structure) Enzymes are named for the substrate that they work with. Names usually end in –ase (ex. Lactase, He ...
Circular dichroism

Circular dichroism (CD) is dichroism involving circularly polarized light, i.e., the differential absorption of left- and right-handed light. Left-hand circular (LHC) and right-hand circular (RHC) polarized light represent two possible spin angular momentum states for a photon, and so circular dichroism is also referred to as dichroism for spin angular momentum. This phenomenon was discovered by Jean-Baptiste Biot, Augustin Fresnel, and Aimé Cotton in the first half of the 19th century. It is exhibited in the absorption bands of optically active chiral molecules. CD spectroscopy has a wide range of applications in many different fields. Most notably, UV CD is used to investigate the secondary structure of proteins. UV/Vis CD is used to investigate charge-transfer transitions. Near-infrared CD is used to investigate geometric and electronic structure by probing metal d→d transitions. Vibrational circular dichroism, which uses light from the infrared energy region, is used for structural studies of small organic molecules, and most recently proteins and DNA.