unit_k_reading_notes
... Let us begin with defining stoichiometry. Most texts break it down into 2 categories. The first one we’ve already seen—it’s composition stoichiometry, which is the study of mass relationships of elements in compounds. Examples of this include calculating percentage composition, and determination of ...
... Let us begin with defining stoichiometry. Most texts break it down into 2 categories. The first one we’ve already seen—it’s composition stoichiometry, which is the study of mass relationships of elements in compounds. Examples of this include calculating percentage composition, and determination of ...
Key
... lowest boiling = CH3 CH3 < CH3 Cl < CH3 SH < CH3 OH = highest boiling −89 ◦ C < −23.8 ◦ C < 5.95 ◦ C < 64.7 ◦ C C2 H6 has no dipole and the smallest London forces; it must be the lowest boiling. Strong hydrogen bonding in CH3 OH will make it the highest boiling. For CH3 Cl and CH3 SH, London forces ...
... lowest boiling = CH3 CH3 < CH3 Cl < CH3 SH < CH3 OH = highest boiling −89 ◦ C < −23.8 ◦ C < 5.95 ◦ C < 64.7 ◦ C C2 H6 has no dipole and the smallest London forces; it must be the lowest boiling. Strong hydrogen bonding in CH3 OH will make it the highest boiling. For CH3 Cl and CH3 SH, London forces ...
Chapters Study Guide
... 2. Limiting reactant problems (aka calculate theoretical yield) “In the reaction CaC2 + 2H2O C2H2 + Ca(OH)2, 64 g H2O is reacted with 64 g CaC2. CaC2. Which is the excess reactant, which is limiting? What is the theoretical yield of C 2H2 ? ...
... 2. Limiting reactant problems (aka calculate theoretical yield) “In the reaction CaC2 + 2H2O C2H2 + Ca(OH)2, 64 g H2O is reacted with 64 g CaC2. CaC2. Which is the excess reactant, which is limiting? What is the theoretical yield of C 2H2 ? ...
Physical chemistry - MCQ topic quiz
... Chemistry teaching and learning resources, including delivery guides, topic exploration packs, lesson elements and more are available on the qualification webpages. If you are looking for examination practice materials, you can find Sample Assessment Materials (SAMs) and a link to the Practice Paper ...
... Chemistry teaching and learning resources, including delivery guides, topic exploration packs, lesson elements and more are available on the qualification webpages. If you are looking for examination practice materials, you can find Sample Assessment Materials (SAMs) and a link to the Practice Paper ...
Percent Ionization
... - a solution characterized by the ability to resist changes in pH when limited amounts of acid or base are added to it - contain either a weak acid and its conjugate base or a base and it conjugate acid - so, buffer solution contains both an acid species and a base species in equilibrium Consider bu ...
... - a solution characterized by the ability to resist changes in pH when limited amounts of acid or base are added to it - contain either a weak acid and its conjugate base or a base and it conjugate acid - so, buffer solution contains both an acid species and a base species in equilibrium Consider bu ...
45. kinetics ch 12
... When stoichiometric relationships in a chemical reaction are not one to one, the molar ratio must be applied to determining reaction rates. For a general reaction, aA + bB → cC + dD, the reaction velocity (reaction rate) can be written in a number of different but equivalent ways: ...
... When stoichiometric relationships in a chemical reaction are not one to one, the molar ratio must be applied to determining reaction rates. For a general reaction, aA + bB → cC + dD, the reaction velocity (reaction rate) can be written in a number of different but equivalent ways: ...
Honors Chemistry
... An increase in pressure will shift the reaction to the A decrease in pressure will shift the reaction to the Please note: Keq ...
... An increase in pressure will shift the reaction to the A decrease in pressure will shift the reaction to the Please note: Keq ...
File
... Spring 2012, Lecture test II in chemistry 2. Name___________________________ Answer questions 1 to 19 on these pages. ______1. Which of the following will increase the Ksp of PbCl2 ? A) Addition of HCl to the solution B) Addition of Pb(NO3)2 to the solution C) An increase in temperature D) All of th ...
... Spring 2012, Lecture test II in chemistry 2. Name___________________________ Answer questions 1 to 19 on these pages. ______1. Which of the following will increase the Ksp of PbCl2 ? A) Addition of HCl to the solution B) Addition of Pb(NO3)2 to the solution C) An increase in temperature D) All of th ...
The EMF technique
... I d I tot dx I n zne dµn I e dµe • Under open circuit conditions, using equal, intert electrodes, we get: II ...
... I d I tot dx I n zne dµn I e dµe • Under open circuit conditions, using equal, intert electrodes, we get: II ...
• • • • • • • • • • • • • • • • • • • • • • • • • •
... Define the properties of acids and bases. Compare and contrast acids and bases as defined by the theories of Arrhenius, Bronsted-Lowry, and Lewis. 5. Hydrogen Ions and Acidity Describe how [H+] and [OH-] are related in an aqueous solution. Classify a solution as neutral, acidic, or basic giv ...
... Define the properties of acids and bases. Compare and contrast acids and bases as defined by the theories of Arrhenius, Bronsted-Lowry, and Lewis. 5. Hydrogen Ions and Acidity Describe how [H+] and [OH-] are related in an aqueous solution. Classify a solution as neutral, acidic, or basic giv ...
Calculating the conductivity of natural waters
... conductivities for all constituents and then scaling by a specified function of ionic strength. This provides good results for North American fresh waters. Sorensen and Glass (1987) assumed constancy of the product of viscosity and a power of conductivity (a modified Walden product) and developed a ...
... conductivities for all constituents and then scaling by a specified function of ionic strength. This provides good results for North American fresh waters. Sorensen and Glass (1987) assumed constancy of the product of viscosity and a power of conductivity (a modified Walden product) and developed a ...
AP Chemistry: Total Notes Review
... o Elements in the same group on the Periodic Table have the same type of electron arrangement in their outermost shells ex) F, [He]2s22p5; and Cl[Ne]3s23p5 ~ Outer-shell electrons: those that lie outside the orbitals occupied in the nextlowest noble gas element ex)[He]2s22p5 ~ Valence electrons: out ...
... o Elements in the same group on the Periodic Table have the same type of electron arrangement in their outermost shells ex) F, [He]2s22p5; and Cl[Ne]3s23p5 ~ Outer-shell electrons: those that lie outside the orbitals occupied in the nextlowest noble gas element ex)[He]2s22p5 ~ Valence electrons: out ...
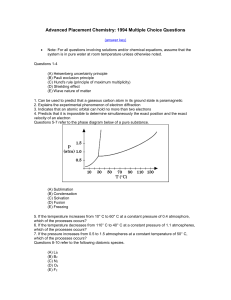
1994 Released Exam
... 15 In a moleculein which the central atom exhibits sp3d2 hybrid orbitals,the electronpairs are directed ...
... 15 In a moleculein which the central atom exhibits sp3d2 hybrid orbitals,the electronpairs are directed ...
pH scale. Buffer solutions. Colligative properties of solutions
... If рН value of the blood is 7.40, the ratio of the concentrations of the ions [HPO42−]/[H2PO4−] is 1.55 : 1. In the erythrocytes the pH is 7.25, the principal buffer systems are bicarbonate HCO3−/H2CO3 and hemoglobin systems. As a very rough approximation, we can treat it as a weak monoprotic acid o ...
... If рН value of the blood is 7.40, the ratio of the concentrations of the ions [HPO42−]/[H2PO4−] is 1.55 : 1. In the erythrocytes the pH is 7.25, the principal buffer systems are bicarbonate HCO3−/H2CO3 and hemoglobin systems. As a very rough approximation, we can treat it as a weak monoprotic acid o ...