* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download 45. kinetics ch 12
Electrochemistry wikipedia , lookup
Stability constants of complexes wikipedia , lookup
Marcus theory wikipedia , lookup
Chemical thermodynamics wikipedia , lookup
Woodward–Hoffmann rules wikipedia , lookup
Equilibrium chemistry wikipedia , lookup
Ene reaction wikipedia , lookup
Determination of equilibrium constants wikipedia , lookup
Supramolecular catalysis wikipedia , lookup
Ultraviolet–visible spectroscopy wikipedia , lookup
Industrial catalysts wikipedia , lookup
Chemical equilibrium wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Enzyme catalysis wikipedia , lookup
George S. Hammond wikipedia , lookup
Reaction progress kinetic analysis wikipedia , lookup
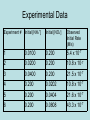
Chemical Kinetics The rate of a reaction is the positive quantity that expresses how the concentration of a reactant or product changes with time. The rates of reactions span an enormous range. From those that are complete within seconds to those that take thousands or even millions of years. Factors that effect reaction rates... The physical state of the reactants: The ability of the reactants to collide lead to a reaction. The more readily molecules collide with each other, the more rapidly they react. A solid that is broken in up in to pieces will react faster than one that is not because of a greater surface area for reactions. The concentrations of reactants: Most chemical reactions occur faster if the concentration of the reactants is increased. A higher concentration calls for more collisions, and more collisions lead to a faster reaction. The temperature at which the reaction occurs: The rates of chemical reactions increase as temperature is increased. An increase in temperature leads to higher kinetic energy of the substance and more collisions for the reactions to take place faster. The presence of a catalyst: Catalysts are agents that increase reaction rates without being used up. They affect the kinds of collisions (mechanisms) that lead up to a reaction. Collisions, Collisions, Collisions On a molecular level: Rates depend on the frequency of the collisions between molecules, the greater the frequency of collisions, the greater the rate of reaction. Reaction Rates A reaction rate is the change in the concentration of reactants or products per unit time. The unit for a reaction rate is then molarity per seconds (M/s). The rate of a reaction can be based on the rate of disappearance of a reactant of the rate of appearance for a product. Example: A B Time (s) [A] [B] 0.00 1.00 0.00 20 0.54 0.46 40 0.30 0.70 • Rate of appearance of B = (D[B]) (Dt) • Average rate = 0.46M – 0.00M = 2.3 x 10-2 M/s 20 s – 0.0 s Rates of Reaction & Stoich. When stoichiometric relationships in a chemical reaction are not one to one, the molar ratio must be applied to determining reaction rates. For a general reaction, aA + bB → cC + dD, the reaction velocity (reaction rate) can be written in a number of different but equivalent ways: Example: Example: 2HI (g) H2 (g) + I2 (g) Rate = - 1 D[HI] = D[H2] = D[I2] 2 Dt Dt Dt The Rate Law In general the rate law expression for the general reaction: aA + bB → cC + dD is Rate = k[A]m[B]n Rate = M/s k = units will vary depending on the rate order of the reactants [A] & [B] = M m & n = will be whole numbers that determine the rate order of the reactants, they can vary and are no way related to the coefficients. Reaction Rates & Concentration One way of studying the effect of concentration on reaction rate is to determine the way in which the rate at the beginning of a reaction depends on the starting concentrations. Let us consider the following reaction to illustrate: NH4+ (aq) + NO2- (aq) N2 (g) + 2H2O (l) Experimental Data Experiment # Initial [NH4+] Initial [NO2-] Observed Initial Rate (M/s) 1 0.0100 0.200 5.4 x 10-7 2 0.0200 0.200 10.8 x 10-7 3 0.0400 0.200 21.5 x 10-7 4 0.200 0.0202 10.8 x 10-7 5 0.200 0.0404 21.6 x 10-7 6 0.200 0.0808 43.3 x 10-7 Rate Order 0 order: The concentration of the reactants has no impact on the rate of the reaction. 1st order: The rate doubles when concentration is doubles ([2]1 = 2), , triples when concentration is tripled ([3]1 = 3), quadruples when the concentration is quadrupled ([4]1 = 4), and so forth (raised to the 1st power in the rate law). 2nd order: The rate quadruples when the concentration is doubled ([2]2 = 4), the rate increases ninefold when the concentration is triples ([3]2 = 9), and so forth (raised to the 2nd power in the rate law). Rate Law Expression • The rate law expression for this reaction is: Rate = k[NH4+]1[NO2-]1 – take note only reactants appear in the rate law expression – k represents a proportionality constant called the rate law constant (sometimes simply the rate constant). – overall rate order for the reaction is the sum of the rate orders for the reactants. So for the above reaction the rate order is 2. • Example: Determine the rate law constant for this reaction. Once the rate law constant is determined the rate could be calculated at any reactant concentration. Example: Determine the rate of the reaction when [NH4+] = 2.13M and [NO2-] = 0.986M. 1st Order Rate Law • Where X is the concentration of a reactant at any moment in time, (X)o is the initial concentration of this reactant, k is the constant for the reaction, and t is the time since the reaction started. • This equation is useful in calculating how much of a substance remains after a certain amount of time has passed, or to calculate how long it takes until the concentration is at a certain point. First Order Reactions If the rate law of a reaction is first order with respect to [A], then the graph of ln[A] versus time (t) creates a straight line with a negative slope. The value of the slope of the line is equal to the negative value of the rate constant (k). Half-lives are 1st Order The equation for the half-life of a substance is derived from this equation. Half-life - The length of time it takes for exactly half of the nuclei of a radioactive sample to decay. 2nd Order Rate Law • Where X is the concentration of a reactant at any moment in time, (X)o is the initial concentration of this reactant, k is the constant for the reaction, and t is the time since the reaction started. • This equation is useful in calculating how much of a substance remains after a certain amount of time has passed, or to calculate how long it takes until the concentration is at a certain point. Second Order Reactions If the rate law for a reaction is second order with respect to [A], a graph of 1/[A] versus time (t) creates a straight line with a positive slope. The value of the slope of the line is equal to the value of the rate constant (k). MC #1 Relatively slow rates of chemical reaction are associated with which of the following? (A) The presence of a catalyst (B) High temperature (C) High concentration of reactants (D) Strong bonds in reactant molecules (E) Low activation energy MC #2 The proposed steps for a catalyzed reaction between Ce4+ and Tl+ are represented above. The products of the overall catalyzed reaction are Step 1: Ce4+ + Mn2+ ---> Ce3+ + Mn3+ Step 2: Ce4+ + Mn3+ ---> Ce3+ + Mn4+ Step 3: Mn4+ + Tl+ ---> Tl3+ + Mn2+ (A) Ce4+ and Tl+ (B) Ce3+ and Tl3+ (C) Ce3+ and Mn3+ (D) Ce3+ and Mn4+ (E) Tl3+ and Mn2+ MC #3 (CH3)3CCl(aq) + OH¯ ---> (CH3)3COH(aq) + Cl¯ For the reaction represented above, the experimental rate law is given as follows. Rate = k [(CH3)3CCl] If some solid sodium solid hydroxide is added to a solution that is 0.010-molar in (CH3)3CCl and 0.10-molar in NaOH, which of the following is true? (Assume the temperature and volume remain constant.) (A) Both the reaction rate and k increase. (B) Both the reaction rate and k decrease. (C) Both the reaction rate and k remain the same. (D) The reaction rate increases but k remains the same. (E) The reaction rate decreases but k remains the same. MC #4 & 5 Questions 4 & 5: H3AsO4 + 3I¯ + 2 H3O+ ---> H3AsO3 + I3¯ + H2O The oxidation of iodide ions by arsenic acid in acidic aqueous solution occurs according to the stoichiometry shown above. The experimental rate law of the reaction is: Rate = k [H3AsO4] [I¯] [H3O+] 4. What is the order of the reaction with respect to I¯? (A) 1 (B) 2 (C) 3 (D) 5 (E) 6 5. According to the rate law for the reaction, an increase in the concentration of hydronium ion has what effect on this reaction? (A) The rate of reaction increases. (B) The rate of reaction decreases. (C) The value of the equilibrium constant increases. (D) The value of the equilibrium constant decreases. (E) Neither the rate nor the value of the equilibrium constant is changed. FRQ #1: slide 1 A(g) + B(g) C(g) + D(g) • For the gas-phase reaction represented above, the following experimental data were obtained: Experiment Initial [A] (mol L-1) Initial [B] (mol L-1) Initial Reaction Rate (mol L-1 s-l) 1 2 3 4 0.033 0.034 0.136 0.202 0.034 0.137 0.136 0.233 6.6710-4 1.0810-2 1.0710-2 ? FRQ #1: slide 2 (a) Determine the order of the reaction with respect to reactant A. Justify your answer. (b) Determine the order of the reaction with respect to reactant B. Justify your answer. (c) Write the rate law for the overall reaction. (d) Determine the value of the rate constant, k, for the reaction. Include units with your answer. (e) Calculate the initial reaction rate for experiment 4. (f) The following mechanism has been proposed for the reaction. Step 1: B + B E + D slow Step 2: E + A B + C fast equilibrium • Provide two reasons why the mechanism is acceptable. (g) In the mechanism in part (f), is species E a catalyst, or is it an intermediate? Justify your answer.