Turbomachinery
... • 1st Law of Thermo [Conservation of energy]: Total work is same in all adiabatic processes between any two equilibrium states having same kinetic and potential energy. – Introduces idea of stored or internal energy E – dE = dQ - dW • dW = Work done by system [+]=dWout= - pdV • Some books have dE=dQ ...
... • 1st Law of Thermo [Conservation of energy]: Total work is same in all adiabatic processes between any two equilibrium states having same kinetic and potential energy. – Introduces idea of stored or internal energy E – dE = dQ - dW • dW = Work done by system [+]=dWout= - pdV • Some books have dE=dQ ...
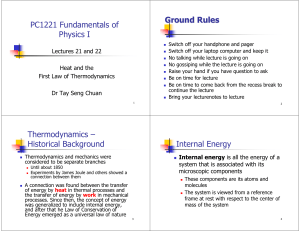
Lectures 21 and 22 - NUS Physics Department
... Thermodynamics and mechanics were considered to be separate branches ...
... Thermodynamics and mechanics were considered to be separate branches ...
Energy - NTOU-Chem
... and Work The firing of a potato cannon provides a good example of the heat and work associated with a chemical reaction. A potato is stuffed into a long cylinder that is capped on one end and open at the other. Some kind of fuel is introduced under the potato at the capped end—usually through a smal ...
... and Work The firing of a potato cannon provides a good example of the heat and work associated with a chemical reaction. A potato is stuffed into a long cylinder that is capped on one end and open at the other. Some kind of fuel is introduced under the potato at the capped end—usually through a smal ...
Thermodynamic Concep..
... Now we have to come back to the definition of entropy change in terms of heat and temperature. As long as we agree to keep the temperature of the surroundings constant, we can arrange things so that heat is transferred to it or from it in a reversible fashion. This concept of reversible is kind of t ...
... Now we have to come back to the definition of entropy change in terms of heat and temperature. As long as we agree to keep the temperature of the surroundings constant, we can arrange things so that heat is transferred to it or from it in a reversible fashion. This concept of reversible is kind of t ...
Document
... between the temperature of reservoir and the temperature of the system (vapor + water) which is lowered because of the endothermic nature of the evaporation process. This temperature gradient forces a heat transfer from the reservoir to the content of the cylinder to reestablish thermal equilibrium ...
... between the temperature of reservoir and the temperature of the system (vapor + water) which is lowered because of the endothermic nature of the evaporation process. This temperature gradient forces a heat transfer from the reservoir to the content of the cylinder to reestablish thermal equilibrium ...
Thermochemistry
... Cp is the amount of heat needed to change a unit mass of material by a unit temperature change. It is called the specific heat capacity. Most commonly the units are J/gC° or J/mole C°. The value of Cp changes from one phase to another and from one substance to another. The subscript p refers to the ...
... Cp is the amount of heat needed to change a unit mass of material by a unit temperature change. It is called the specific heat capacity. Most commonly the units are J/gC° or J/mole C°. The value of Cp changes from one phase to another and from one substance to another. The subscript p refers to the ...
Chapter 15 Lesson 2
... OF THE SECOND LAW OF THERMODYNAMICS No irreversible engine operating between two reservoirs at constant Temperatures can have a greater efficiency than a reversible engine operating between the same temperatures. Furthermore, all reversible engines operating between the same temperatures have the sa ...
... OF THE SECOND LAW OF THERMODYNAMICS No irreversible engine operating between two reservoirs at constant Temperatures can have a greater efficiency than a reversible engine operating between the same temperatures. Furthermore, all reversible engines operating between the same temperatures have the sa ...
fake sem 2 unit 1 test.tst
... Multiple Choice Identify the choice that best completes the statement or answers the question. ____ ...
... Multiple Choice Identify the choice that best completes the statement or answers the question. ____ ...
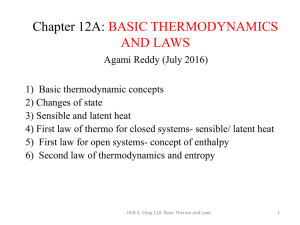
Chap-12A_Basic-Thermo-and-Laws
... (b) Specific internal energy u refers to the energy stored by a substance due to the microscopic motion and/or position of the molecules. This form of energy consists of two parts: the internal kinetic energy due to the velocity of the molecules, and the internal potential energy due to the attracti ...
... (b) Specific internal energy u refers to the energy stored by a substance due to the microscopic motion and/or position of the molecules. This form of energy consists of two parts: the internal kinetic energy due to the velocity of the molecules, and the internal potential energy due to the attracti ...
Material and Energy Balances CHEN 2120 Outline Specific
... • “Designed” to take into account ‘PV’ work for open systems • Example: If the specific internal energy of a gas at 300 K and 1 atm is 4200 J/mol, and the specific molar volume at the same temperature and pressure is 25.34 L/mol, the specific enthalpy of the gas is: ...
... • “Designed” to take into account ‘PV’ work for open systems • Example: If the specific internal energy of a gas at 300 K and 1 atm is 4200 J/mol, and the specific molar volume at the same temperature and pressure is 25.34 L/mol, the specific enthalpy of the gas is: ...
GCE Physics - Thermodynamics Notes Word Document
... A chemical reaction occurs and the contents of the vessel turn wholly or partly to steam. There is a sudden huge rise in the temperature and pressure in the vessel. What has happened to the internal energy of the system (i.e. the contents of the vessel)? Has heat flowed into or out of the system? Ha ...
... A chemical reaction occurs and the contents of the vessel turn wholly or partly to steam. There is a sudden huge rise in the temperature and pressure in the vessel. What has happened to the internal energy of the system (i.e. the contents of the vessel)? Has heat flowed into or out of the system? Ha ...