Document
... Adiabatic: No heat is transferred into or out of the system, so Q = 0. Also, U2 – U1 = –W. Isochoric: The volume remains constant, so W = 0. Isobaric: The pressure remains constant, so W = p(V2 – V1). Isothermal: The temperature remains constant. ...
... Adiabatic: No heat is transferred into or out of the system, so Q = 0. Also, U2 – U1 = –W. Isochoric: The volume remains constant, so W = 0. Isobaric: The pressure remains constant, so W = p(V2 – V1). Isothermal: The temperature remains constant. ...
Thermodynamics: C l i t H t alorimetry, Heat
... • A process refers to the change of a system from one equilibrium state to another. • The initial and final states of a process are its end-points. end points • A quasistatic process is one that takes place so slowly that th system the t may be b considered id d as passing i th through ha succession ...
... • A process refers to the change of a system from one equilibrium state to another. • The initial and final states of a process are its end-points. end points • A quasistatic process is one that takes place so slowly that th system the t may be b considered id d as passing i th through ha succession ...
Chemical Thermodynamics John Murrell Introduction
... Atoms and molecules have energies and we can relate these to the macroscopic energies that arise in thermodynamics; for this discussion we will ignore the difference between U and H, which we have seen is generally small. For a low pressure gas we can multiply the molecular energy by Avogadro’s con ...
... Atoms and molecules have energies and we can relate these to the macroscopic energies that arise in thermodynamics; for this discussion we will ignore the difference between U and H, which we have seen is generally small. For a low pressure gas we can multiply the molecular energy by Avogadro’s con ...
Chapter 1 Energy Accounting, Variables and Properties of Systems
... train rolls across the European continent. While it was understood at that time that steam engines essentially convert heat to mechanical work, no quantitative theory was known to predict conversion efficiencies, and heat was not quite yet seen as a form of energy. It is out of the practical enginee ...
... train rolls across the European continent. While it was understood at that time that steam engines essentially convert heat to mechanical work, no quantitative theory was known to predict conversion efficiencies, and heat was not quite yet seen as a form of energy. It is out of the practical enginee ...
Chapter 1
... Equilibrium: In thermodynamics, the concept of equilibrium includes not only a balance of forces but also a balance of other influencing factors, such as thermal equilibrium, pressure equilibrium, phase equilibrium, etc. Zeroth law of thermodynamics is law of thermal equilibrium, which states that if ...
... Equilibrium: In thermodynamics, the concept of equilibrium includes not only a balance of forces but also a balance of other influencing factors, such as thermal equilibrium, pressure equilibrium, phase equilibrium, etc. Zeroth law of thermodynamics is law of thermal equilibrium, which states that if ...
Chapter 1 - All Made Easy
... Equilibrium: In thermodynamics, the concept of equilibrium includes not only a balance of forces but also a balance of other influencing factors, such as thermal equilibrium, pressure equilibrium, phase equilibrium, etc. Zeroth law of thermodynamics is law of thermal equilibrium, which states that if ...
... Equilibrium: In thermodynamics, the concept of equilibrium includes not only a balance of forces but also a balance of other influencing factors, such as thermal equilibrium, pressure equilibrium, phase equilibrium, etc. Zeroth law of thermodynamics is law of thermal equilibrium, which states that if ...
Humphrey, Tammy - Quantum Electronics Group
... 2) The difference between the Carnot limit and the actual efficiency of a practical heat engine does not therefore represent a ‘true’ measure of the efficiency gain which might be achieved with further optimization ...
... 2) The difference between the Carnot limit and the actual efficiency of a practical heat engine does not therefore represent a ‘true’ measure of the efficiency gain which might be achieved with further optimization ...
Carnot Cycle. Heat Engines. Refrigerators.
... We have found a limit on the efficiency of an engine. How good is this limit? In other words, is the upper bound on efficiency attainable? From our derivation of the upper bound it is clear that to attain it one must create no net entropy in the environment after each cycle. In particular, the entro ...
... We have found a limit on the efficiency of an engine. How good is this limit? In other words, is the upper bound on efficiency attainable? From our derivation of the upper bound it is clear that to attain it one must create no net entropy in the environment after each cycle. In particular, the entro ...
WRL0638.tmp - Symposium on Chemical Physics
... ‘therefore’ be viewed to be equilibrium states. It does not seem possible to give an unambiguous definition of an equilibrium state vs. metastable state. Equilibrium states are defined in the sense that they satisfy the postulates of equilibrium thermodynamics! (HC). Since the equilibrium states are ...
... ‘therefore’ be viewed to be equilibrium states. It does not seem possible to give an unambiguous definition of an equilibrium state vs. metastable state. Equilibrium states are defined in the sense that they satisfy the postulates of equilibrium thermodynamics! (HC). Since the equilibrium states are ...
Fundamentals of Equilibrium Thermodynamics
... ‘therefore’ be viewed to be equilibrium states. It does not seem possible to give an unambiguous definition of an equilibrium state vs. metastable state. Equilibrium states are defined in the sense that they satisfy the postulates of equilibrium thermodynamics! (HC). Since the equilibrium states are ...
... ‘therefore’ be viewed to be equilibrium states. It does not seem possible to give an unambiguous definition of an equilibrium state vs. metastable state. Equilibrium states are defined in the sense that they satisfy the postulates of equilibrium thermodynamics! (HC). Since the equilibrium states are ...
Thermodynamics
... Q = quantity of heat [can also be a small q or )H for a chemical reaction] m = mass [watch the units--could be g, could be kg, could be moles where “n” is used instead of “m”] )T = change in temperature; final - initial BUT not always! Q ( +) if heat flows IN Q (-) if heat flows OUT Why isn’t )T not ...
... Q = quantity of heat [can also be a small q or )H for a chemical reaction] m = mass [watch the units--could be g, could be kg, could be moles where “n” is used instead of “m”] )T = change in temperature; final - initial BUT not always! Q ( +) if heat flows IN Q (-) if heat flows OUT Why isn’t )T not ...
College Physics II - Tennessee State University
... ten (10) points for a total of 300 points. Other instructions will be given in the EXAM HALL, if necessary. ...
... ten (10) points for a total of 300 points. Other instructions will be given in the EXAM HALL, if necessary. ...
AOSS_401_20070919_L06_Thermo_Energy
... • We have formed equations to predict changes in motion (conservation of momentum) and density (conservation of mass) • We need one more equation to describe either the time rate of change of pressure or temperature (they are linked through the ideal gas law) • Conservation of energy is the basic pr ...
... • We have formed equations to predict changes in motion (conservation of momentum) and density (conservation of mass) • We need one more equation to describe either the time rate of change of pressure or temperature (they are linked through the ideal gas law) • Conservation of energy is the basic pr ...
Energy
... can be done in the reverse direction. For example, when a liquid evaporates its volume expands and can push on a piston to do work on the outside. This is the basis of the steam engine. P is the force, and the change in V is the displacement. As we have noted above, (P, V ) forms a conjugate pair. W ...
... can be done in the reverse direction. For example, when a liquid evaporates its volume expands and can push on a piston to do work on the outside. This is the basis of the steam engine. P is the force, and the change in V is the displacement. As we have noted above, (P, V ) forms a conjugate pair. W ...
ME 204 Thermodynamics I
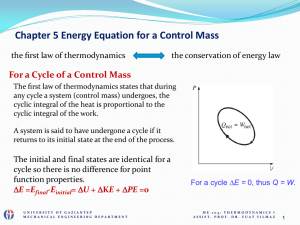
... iv) Is it helpful to draw a diagram of the information (for example, a T-v or P-v diagram)? (may show the process and states) v) What is our thermodynamic model for the behavior of the substance (for example, steam tables, ideal gas, and so on)? (the methods to drive termodynamic properties) vi) Wh ...
... iv) Is it helpful to draw a diagram of the information (for example, a T-v or P-v diagram)? (may show the process and states) v) What is our thermodynamic model for the behavior of the substance (for example, steam tables, ideal gas, and so on)? (the methods to drive termodynamic properties) vi) Wh ...