2.2 Thermoelasticity
... In this section, thermoelasticity is considered. By definition, the constitutive relations for such a material depend only on the set of field variables F, , Grad . This general case will be considered toward the end of this section. Simpler cases, isothermal F , smallstrain ε, one-dimension ...
... In this section, thermoelasticity is considered. By definition, the constitutive relations for such a material depend only on the set of field variables F, , Grad . This general case will be considered toward the end of this section. Simpler cases, isothermal F , smallstrain ε, one-dimension ...
Document
... temperature region TL. An amount of heat QH = QL + W is then removed from the system at a higher temperature TH. (a) Represents the cylinder in a car engine; while (b) represents a ...
... temperature region TL. An amount of heat QH = QL + W is then removed from the system at a higher temperature TH. (a) Represents the cylinder in a car engine; while (b) represents a ...
Does the Third Law of Thermodynamics Hold
... In recent years, the realization that quantum effects can play an important role in thermodynamic theory has led to an intense interest in quantum and mesoscopic thermodynamics,(1–5) to the extent that some authors(1–4) have questioned whether the fundamental laws of thermodynamics remain valid. The ...
... In recent years, the realization that quantum effects can play an important role in thermodynamic theory has led to an intense interest in quantum and mesoscopic thermodynamics,(1–5) to the extent that some authors(1–4) have questioned whether the fundamental laws of thermodynamics remain valid. The ...
Chapter 13 Thermodynamics (mostly Chapter 19)
... freedom was usually small. For N ! 10 analytical methods may be useful; for N ! 1010 computer may work; for N ∼ 1 googol = 10100 statistical physics may be the only tool. There are two standard ways to study the large N limit: • phenomenological (e.g. thermodynamics) and • fundamental (e.g. statisti ...
... freedom was usually small. For N ! 10 analytical methods may be useful; for N ! 1010 computer may work; for N ∼ 1 googol = 10100 statistical physics may be the only tool. There are two standard ways to study the large N limit: • phenomenological (e.g. thermodynamics) and • fundamental (e.g. statisti ...
Chapter 14 The Ideal Gas Law and Kinetic Theory
... Walls that permit heat flow are called diathermal walls, while walls that do not permit heat flow are called adiabatic (NO HEAT FLOW) walls To understand thermodynamics, it is necessary to describe the state of a system. ...
... Walls that permit heat flow are called diathermal walls, while walls that do not permit heat flow are called adiabatic (NO HEAT FLOW) walls To understand thermodynamics, it is necessary to describe the state of a system. ...
Thermodynamics of ideal gases
... stay constant. Reversibility is an ideal which can only be approached by very slow quasistatic processes, consisting of infinitely many infinitesimal reversible steps. Essentially all real-world processes are irreversible to some degree. Example D.3.1 (Joule’s expansion experiment): An isolated box ...
... stay constant. Reversibility is an ideal which can only be approached by very slow quasistatic processes, consisting of infinitely many infinitesimal reversible steps. Essentially all real-world processes are irreversible to some degree. Example D.3.1 (Joule’s expansion experiment): An isolated box ...
First law of thermodynamics
... State functions are characterized by the idea that no matter what path you take to get from point A to point B, the difference between B and A remains the same. An example of how this works can be illustrated using gravitational potential energy. Say you lift a box 5 meters vertically straight up in ...
... State functions are characterized by the idea that no matter what path you take to get from point A to point B, the difference between B and A remains the same. An example of how this works can be illustrated using gravitational potential energy. Say you lift a box 5 meters vertically straight up in ...
5.27 MB - KFUPM Resources v3
... molar flow rate and volumetric flow rate for a continuous stream), and kinetic energy, potential energy, and internal energy (or the rates of transport of these quantities by a continuous stream) are extensive properties Intensive: temperature, pressure, and density ...
... molar flow rate and volumetric flow rate for a continuous stream), and kinetic energy, potential energy, and internal energy (or the rates of transport of these quantities by a continuous stream) are extensive properties Intensive: temperature, pressure, and density ...
First Law of Thermodynamics Heat and Work done by a Gas
... place to keep the volume constant. The weight of the piston can be adjusted to adjust the pressure as necessary. Starting with the same initial conditions, you do three experiments, each involving adding the same amount of heat, Q. A – Add the heat at constant pressure. B – Add the heat at constant ...
... place to keep the volume constant. The weight of the piston can be adjusted to adjust the pressure as necessary. Starting with the same initial conditions, you do three experiments, each involving adding the same amount of heat, Q. A – Add the heat at constant pressure. B – Add the heat at constant ...
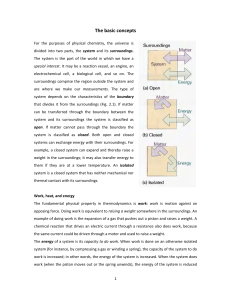
The basic concepts For the purposes of physical chemistry, the
... and it can do less work than before. Experiments have shown that the energy of a system may be changed by means other than work itself. When the energy of a system changes as a result of a temperature difference between the system and its surroundings we say that energy has been transferred as heat ...
... and it can do less work than before. Experiments have shown that the energy of a system may be changed by means other than work itself. When the energy of a system changes as a result of a temperature difference between the system and its surroundings we say that energy has been transferred as heat ...