File - SRIT - MECHANICAL ENGINEERING
... The term internal energy usually denoted by the letter U is the energy due to such factors as electron spin and vibrations, molecular motion and chemical bond. Kinetic energy term is due to the system movement with a velocity C. For stationary systems this term will be zero. The term gc is a constan ...
... The term internal energy usually denoted by the letter U is the energy due to such factors as electron spin and vibrations, molecular motion and chemical bond. Kinetic energy term is due to the system movement with a velocity C. For stationary systems this term will be zero. The term gc is a constan ...
ME 435: Thermal Energy Systems Design
... In past lectures we found that the power draw (Wc) and the capacity (Qe) are important performance parameters for a compressor. If we can develop heat exchanger models that describe the heat transfer rate, we have a set of equations that are coupled together. For example, the evaporator heat transfe ...
... In past lectures we found that the power draw (Wc) and the capacity (Qe) are important performance parameters for a compressor. If we can develop heat exchanger models that describe the heat transfer rate, we have a set of equations that are coupled together. For example, the evaporator heat transfe ...
Chapter 4 Entropy and second law of thermodynamics
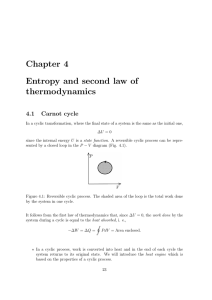
... the same place. This situation can be defined as ”ordered” since there is a strong correlation between the kids’ positions. For example, if Peter sits in the first row, you know straight away who is sitting behind and besides him: there is only one possibility (multiplicity is very low). Looking now ...
... the same place. This situation can be defined as ”ordered” since there is a strong correlation between the kids’ positions. For example, if Peter sits in the first row, you know straight away who is sitting behind and besides him: there is only one possibility (multiplicity is very low). Looking now ...
Convective heat transfer
... conduction, the amount of heat entering any region of an object is equal to amount of heat coming out (if this were not so, the temperature would be rising or falling, as thermal energy was tapped or trapped in a region). .Fourier's law The law of heat conduction, also known as Fourier's law, states ...
... conduction, the amount of heat entering any region of an object is equal to amount of heat coming out (if this were not so, the temperature would be rising or falling, as thermal energy was tapped or trapped in a region). .Fourier's law The law of heat conduction, also known as Fourier's law, states ...
Enthalpy In A Box: Teaching Open Vs. Closed System Work Terms
... convected energy terms and instead form part of the net work terms. The benefits of just having internal energy as opposed to internal energy and enthalpy considerations are highlighted. Introduction Enthalpy, somewhat like entropy appears to be one of thermodynamic’s mysterious and abstract propert ...
... convected energy terms and instead form part of the net work terms. The benefits of just having internal energy as opposed to internal energy and enthalpy considerations are highlighted. Introduction Enthalpy, somewhat like entropy appears to be one of thermodynamic’s mysterious and abstract propert ...
heat engine
... Conceptual Example 8 Natural Limits on the Efficiency of a Heat Engine Consider a hypothetical engine that receives 1000 J of heat as input from a hot reservoir and delivers 1000J of work, rejecting no heat to a cold reservoir whose temperature is above 0 K. Decide whether this engine violates the f ...
... Conceptual Example 8 Natural Limits on the Efficiency of a Heat Engine Consider a hypothetical engine that receives 1000 J of heat as input from a hot reservoir and delivers 1000J of work, rejecting no heat to a cold reservoir whose temperature is above 0 K. Decide whether this engine violates the f ...
What is thermodynamics?
... Thermodynamics has many practical uses. It provides the theory to answer the following sorts of practical materials questions:2 o Is it possible to make such-and-such a material and, if so, what processing variables make it possible? o If I have a given material and I put it in service in a particul ...
... Thermodynamics has many practical uses. It provides the theory to answer the following sorts of practical materials questions:2 o Is it possible to make such-and-such a material and, if so, what processing variables make it possible? o If I have a given material and I put it in service in a particul ...
Thermodynamics
... leave or enter the system. Q= 0 by definition U= Q + W U = W only W = - since gas expands hence U < 0 (tem drops) gas loses temp as expands since don’t provide heat to offset work done by gas ...
... leave or enter the system. Q= 0 by definition U= Q + W U = W only W = - since gas expands hence U < 0 (tem drops) gas loses temp as expands since don’t provide heat to offset work done by gas ...
Applied Thermodynamics for Marine Systems Prof. P. K. Das
... differential quantity. The usual symbol could have been dQ, but, instead of that I have used dQ because this is an in exact deferential. This quantity is an in exact differential. Physically, it means that the heat transfer or the amount of heat transfer does not depend on the end states only, but i ...
... differential quantity. The usual symbol could have been dQ, but, instead of that I have used dQ because this is an in exact deferential. This quantity is an in exact differential. Physically, it means that the heat transfer or the amount of heat transfer does not depend on the end states only, but i ...
Vijay Ramani, J. M. Fenton Thermodynamics of Fuel Cells
... standard chemical engineering thermodynamics texts 2. A Quick Review a. The first law of thermodynamics: This law (also called the law of conservation of energy) states “energy may neither be created nor be destroyed, but may be converted from one form to another.” In other words, the total quantity ...
... standard chemical engineering thermodynamics texts 2. A Quick Review a. The first law of thermodynamics: This law (also called the law of conservation of energy) states “energy may neither be created nor be destroyed, but may be converted from one form to another.” In other words, the total quantity ...