Wave Equation - web page for staff
... L. A Gaussian beam of diameter 0.5 cm to e-2 relavtive power density for λ = 0.63 μm is incident on the first lens. The value of L is constained such that the e-2 relative power density locus is contained within the aperture of the second lens. ...
... L. A Gaussian beam of diameter 0.5 cm to e-2 relavtive power density for λ = 0.63 μm is incident on the first lens. The value of L is constained such that the e-2 relative power density locus is contained within the aperture of the second lens. ...
2. NH3 - Huffman Chemistry Website!
... a. What does the number 235 tell you about uranium? _______________________________ b. Write the symbol for this atom using subscripts to show the mass number and atomic number. ...
... a. What does the number 235 tell you about uranium? _______________________________ b. Write the symbol for this atom using subscripts to show the mass number and atomic number. ...
4.IonicCompounds - Gleneaglesunit1and2chemistry2012
... combined with non-metal atoms • Metallic bonds formed when metal atoms combined with metal atoms. • Covalent bonds formed when non-metal atoms combined with non-metal atoms. ...
... combined with non-metal atoms • Metallic bonds formed when metal atoms combined with metal atoms. • Covalent bonds formed when non-metal atoms combined with non-metal atoms. ...
Study Guide 1st Semester
... 32. Where are the alkali metal elements found? How do their electron configurations end? What are some typical behaviors of alkali metals? 33. Where are the alkaline earth metals found? How do their electron configurations end? What are some typical behaviors of alkaline earth metals? 34. What is a ...
... 32. Where are the alkali metal elements found? How do their electron configurations end? What are some typical behaviors of alkali metals? 33. Where are the alkaline earth metals found? How do their electron configurations end? What are some typical behaviors of alkaline earth metals? 34. What is a ...
ppt - University Of Oregon
... Simulate different circuits to give the best resonance Develop a detector ...
... Simulate different circuits to give the best resonance Develop a detector ...
LIST OF TOPICS COVERED DURING THIS COURSE
... LIST OF TOPICS COVERED DURING THIS COURSE The following should serve as a checklist for your notebook. The topics below include all topics that have been covered this semester and are testable on your final exam. These topics should be studied from a variety of source including inclass notes, homewo ...
... LIST OF TOPICS COVERED DURING THIS COURSE The following should serve as a checklist for your notebook. The topics below include all topics that have been covered this semester and are testable on your final exam. These topics should be studied from a variety of source including inclass notes, homewo ...
L 35 Modern Physics [1] - University of Iowa Physics
... classical explanation • According to classical physics, if the intensity of the light is strong enough, enough energy should be absorbed by the electrons to make them pop out • The wavelength of the light should not make a difference. ...
... classical explanation • According to classical physics, if the intensity of the light is strong enough, enough energy should be absorbed by the electrons to make them pop out • The wavelength of the light should not make a difference. ...
Phonons II
... like neutrons, photons, electrons, as if it had a momentum (the crystal momentum) ...
... like neutrons, photons, electrons, as if it had a momentum (the crystal momentum) ...
L 35 Modern Physics [1] Modern Physics
... the atom. • Recall that the orbiting electrons, according to classical ideas, should very quickly radiate away all of its energy • If this were so, then we would observe that atoms emit light over a continuous range of wavelengths (colors) NOT SO! ...
... the atom. • Recall that the orbiting electrons, according to classical ideas, should very quickly radiate away all of its energy • If this were so, then we would observe that atoms emit light over a continuous range of wavelengths (colors) NOT SO! ...
Thursday, March 27, 2008
... Under which conditions does a real gas behave most like an ideal gas? 1. at low temperatures and high pressures 2. at low temperatures and low pressures ...
... Under which conditions does a real gas behave most like an ideal gas? 1. at low temperatures and high pressures 2. at low temperatures and low pressures ...
Chemical Bonding Review
... represented by two or three lines between atoms. For dot diagrams, two or three pairs of dots are between the elements. ...
... represented by two or three lines between atoms. For dot diagrams, two or three pairs of dots are between the elements. ...
Laboratory Pb Name: Date: ______ (1) Measure the mass of a
... (5) Table S in the Chemistry Reference Tables lists the accepted value for the density of lead. Calculate your percent error. Show all work. ...
... (5) Table S in the Chemistry Reference Tables lists the accepted value for the density of lead. Calculate your percent error. Show all work. ...
untitled - PhysRevLett.111.243901
... thickness. For example, with the thinnest sample (2L=l0c 0:44) the reflection enhancement at the target arrival time and the corresponding transmission enhancement were measured to be 100% and 41%, respectively, the best experimental demonstration reported so far (see Fig. S4 in [18]). The enhance ...
... thickness. For example, with the thinnest sample (2L=l0c 0:44) the reflection enhancement at the target arrival time and the corresponding transmission enhancement were measured to be 100% and 41%, respectively, the best experimental demonstration reported so far (see Fig. S4 in [18]). The enhance ...
εn = ε KE + ε PE = ε PE ε PE = ε PE (1 )
... REPULSION which will occur when 2 electrons are placed in the SAME SPATIAL ORBITAL -- such REPULSION would RAISE the energy of the atom This result is summarised in HUND'S RULE: Other things being equal, THE STATE OF LOWEST ENERGY corresponds to the MAXIMUM NUMBER OF UNPAIRED, PARALLEL SPINS Thus th ...
... REPULSION which will occur when 2 electrons are placed in the SAME SPATIAL ORBITAL -- such REPULSION would RAISE the energy of the atom This result is summarised in HUND'S RULE: Other things being equal, THE STATE OF LOWEST ENERGY corresponds to the MAXIMUM NUMBER OF UNPAIRED, PARALLEL SPINS Thus th ...
Light and Energy AP Style
... Calculate the energy released when an electron moves from n= 4 to n=2 in a hydrogen atom. Calculate the energy released when an electron moves from n= 5 to n=3 in a He+1 ion ...
... Calculate the energy released when an electron moves from n= 4 to n=2 in a hydrogen atom. Calculate the energy released when an electron moves from n= 5 to n=3 in a He+1 ion ...
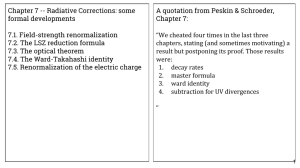
Chapter 7 -- Radiative Corrections: some formal developments Chapter 7:
... W hen it seemed that about hydrogen atom we knew almost everything in 1947 W.E. Lamb and R.C. Retherford decided to check results of Dirac. They used microwaves technique, available from the constructions of radar The Lamb's shift*, a minimal difference in lowest energetic level of the excited hydro ...
... W hen it seemed that about hydrogen atom we knew almost everything in 1947 W.E. Lamb and R.C. Retherford decided to check results of Dirac. They used microwaves technique, available from the constructions of radar The Lamb's shift*, a minimal difference in lowest energetic level of the excited hydro ...
File
... Strong electrolytes- any compound whose dilute aqueous solutions conduct electricity well, due to the presence of all or almost all of the dissolved compound in the form of ions. Weak electrolytes- any compound whose dilute aqueous solutions conduct electricity poorly, due to the presence of a small ...
... Strong electrolytes- any compound whose dilute aqueous solutions conduct electricity well, due to the presence of all or almost all of the dissolved compound in the form of ions. Weak electrolytes- any compound whose dilute aqueous solutions conduct electricity poorly, due to the presence of a small ...
Chemistry Unit Study Guide Key
... or more elements that are bonded together; An element is a pure, single substance made of one type of atom. 2) examples of compounds – CO2, C6H12O6, NaCl, N2, O2, Fe2O3, H2O 3) where metals and nonmetals are found on the periodic table – Metals are to the left of the zig-zag line; Non-metals are to ...
... or more elements that are bonded together; An element is a pure, single substance made of one type of atom. 2) examples of compounds – CO2, C6H12O6, NaCl, N2, O2, Fe2O3, H2O 3) where metals and nonmetals are found on the periodic table – Metals are to the left of the zig-zag line; Non-metals are to ...
Spin Polarized Electron - Jordan University of Science and
... have been tried in attempts to produce beams of spin polarized electrons 1) Scattering from unpolarized target 2) Photoemission from polarized atoms 3) Fano effect 4) The most suitable source photoemission from GaAs. ...
... have been tried in attempts to produce beams of spin polarized electrons 1) Scattering from unpolarized target 2) Photoemission from polarized atoms 3) Fano effect 4) The most suitable source photoemission from GaAs. ...
Chemistry Scavenger Hunt
... 1. All materials, whether solid, liquid or gas, are made of _______________. Atoms are the smallest _______ of ___________. Scientists have found over _______ different kinds of atoms. The many different materials we encounter are made from _______________________ of these atoms. A material in which ...
... 1. All materials, whether solid, liquid or gas, are made of _______________. Atoms are the smallest _______ of ___________. Scientists have found over _______ different kinds of atoms. The many different materials we encounter are made from _______________________ of these atoms. A material in which ...
Rutherford backscattering spectrometry
Rutherford backscattering spectrometry (RBS) is an analytical technique used in materials science. Sometimes referred to as high-energy ion scattering (HEIS) spectrometry, RBS is used to determine the structure and composition of materials by measuring the backscattering of a beam of high energy ions (typically protons or alpha particles) impinging on a sample.









![L 35 Modern Physics [1] - University of Iowa Physics](http://s1.studyres.com/store/data/000679677_1-b925cf8c8f031b0f2b0c09a806312d20-300x300.png)

![L 35 Modern Physics [1] Modern Physics](http://s1.studyres.com/store/data/001558975_1-84d6e03bc786b63795533f59711ce2f4-300x300.png)