1 Chemistry 400: General Chemistry Name: Miller Fall 2015 Final
... involved and their percents of ionization. (8 points) ...
... involved and their percents of ionization. (8 points) ...
Chapter 2 Notes
... A. Physical Properties of Matter = things you can observe without changing a substance; Examples: color; measurements; texture B. Density = measure of the mass of an object divided by its volume; usually given in grams per cubic centimeter (g/cm ); Example: average density of liquid water is 1 g/cm ...
... A. Physical Properties of Matter = things you can observe without changing a substance; Examples: color; measurements; texture B. Density = measure of the mass of an object divided by its volume; usually given in grams per cubic centimeter (g/cm ); Example: average density of liquid water is 1 g/cm ...
Unit 13 Worksheet Answers
... (a) HgO is added to the system increase (c) decrease the temperature of the system. increase (b) Hg is added to the system. decrease (d) the volume is decreased. No change 18) Predict the effect of decreasing the temperature on the position of the following equilibrium. (a) H 2 (g) + Cl 2 (g) ↔ 2HCl ...
... (a) HgO is added to the system increase (c) decrease the temperature of the system. increase (b) Hg is added to the system. decrease (d) the volume is decreased. No change 18) Predict the effect of decreasing the temperature on the position of the following equilibrium. (a) H 2 (g) + Cl 2 (g) ↔ 2HCl ...
Chapter 37 Early Quantum Theory and Models of the Atom
... positive charge, with negative electrons buried throughout. Rutherford did an experiment that showed that the positively charged nucleus must be extremely small compared to the rest of the atom. He scattered alpha particles – helium nuclei – from a metal foil and observed the scattering angle. He fo ...
... positive charge, with negative electrons buried throughout. Rutherford did an experiment that showed that the positively charged nucleus must be extremely small compared to the rest of the atom. He scattered alpha particles – helium nuclei – from a metal foil and observed the scattering angle. He fo ...
Abstract : Fiber interfaces between single atoms and single photons Sébastien Garcia,
... miniaturization, stability et flexibility provided by optical fibers as light wave-guides, we present two experiments where optical fibers are used as interfaces for single atoms trapping and single photons collection into their guided modes. The first experiment combines a singlemode fiber with an ...
... miniaturization, stability et flexibility provided by optical fibers as light wave-guides, we present two experiments where optical fibers are used as interfaces for single atoms trapping and single photons collection into their guided modes. The first experiment combines a singlemode fiber with an ...
Nuclear Chemistry
... An isotope has the same atomic number but different mass number Isotopic notation Mass # ...
... An isotope has the same atomic number but different mass number Isotopic notation Mass # ...
L35 - University of Iowa Physics
... classical explanation • According to classical physics, if the intensity of the light is strong enough, enough energy should be absorbed by the electrons to make them pop out • The wavelength of the light should not make a difference. ...
... classical explanation • According to classical physics, if the intensity of the light is strong enough, enough energy should be absorbed by the electrons to make them pop out • The wavelength of the light should not make a difference. ...
L 35 Modern Physics [1] - University of Iowa Physics
... As speed increases, so does mass Speed can never exceed the speed of light, c ...
... As speed increases, so does mass Speed can never exceed the speed of light, c ...
Chemical Bonding
... are the building blocks of everything. • The subatomic particles that make up atoms are protons, neutrons, and electrons. • Protons=Positive charge • Neutrons=Neutral charge • Electrons=Negative charge ...
... are the building blocks of everything. • The subatomic particles that make up atoms are protons, neutrons, and electrons. • Protons=Positive charge • Neutrons=Neutral charge • Electrons=Negative charge ...
heats of reaction
... HINT: prove/rationalize your answer for CrO3 by writing the equation of the ions coming together to make the product CrO3. Put your suspected charges on the line to MAKE sure you have the correct species! ...
... HINT: prove/rationalize your answer for CrO3 by writing the equation of the ions coming together to make the product CrO3. Put your suspected charges on the line to MAKE sure you have the correct species! ...
Document
... of inelastic collisions between the electrons and the heated mercury gas atoms inside the tube. By being accelerated between the cathode and the anode grid the electrons collect enough energy to energize a mercury gas atom which will afterwards lose its energy by sending out a photon with the same e ...
... of inelastic collisions between the electrons and the heated mercury gas atoms inside the tube. By being accelerated between the cathode and the anode grid the electrons collect enough energy to energize a mercury gas atom which will afterwards lose its energy by sending out a photon with the same e ...
Wavelength
... Why Do We See Different Colors in the Flame Test? A. The electrons are energized to an excited state B. As electrons drop to lower levels, they give off photons C. A photon is a particle of electromagnetic radiation with no mass that carries a quantum of energy D. If the photon’s frequency correspo ...
... Why Do We See Different Colors in the Flame Test? A. The electrons are energized to an excited state B. As electrons drop to lower levels, they give off photons C. A photon is a particle of electromagnetic radiation with no mass that carries a quantum of energy D. If the photon’s frequency correspo ...
Photons and Matter Waves
... a metal surface, a stream of electrons emerges from the metal. The interference filter is then replaced with one transmitting at 300 nm and the lamp adjusted so that the intensity of the light striking the surface is the same as it was for the 400-nm light. With the 300-nm light, 1) more electrons a ...
... a metal surface, a stream of electrons emerges from the metal. The interference filter is then replaced with one transmitting at 300 nm and the lamp adjusted so that the intensity of the light striking the surface is the same as it was for the 400-nm light. With the 300-nm light, 1) more electrons a ...
Modified copy of Flame Tests 2013
... While Rutherford's experimentation and model helped to explain the inner structure of the atom, it did very little to explain the chemical behavior and periodicity of the elements. The next clue toward understanding the nature of atoms was to come from a very different study of chemistry-spectroscop ...
... While Rutherford's experimentation and model helped to explain the inner structure of the atom, it did very little to explain the chemical behavior and periodicity of the elements. The next clue toward understanding the nature of atoms was to come from a very different study of chemistry-spectroscop ...
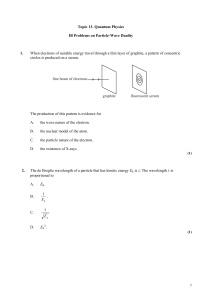
Wave-particle_duality
... The light has intensity 5.0 W m–2. Determine, for an area of 1.0 m2 of the plane surface, (i) ...
... The light has intensity 5.0 W m–2. Determine, for an area of 1.0 m2 of the plane surface, (i) ...
Exam #: Printed Name: Signature: PHYSICS DEPARTMENT
... Find the eigenfunctions and eigenvalues of this system under this Hamiltonian. ...
... Find the eigenfunctions and eigenvalues of this system under this Hamiltonian. ...
atomic physics
... on a string. Higher energy states are then similar to harmonics of the fundamental frequency. 2. The electrons are never in a single point location, although the probability of interacting with the electron at a single point can be found from the wave function of the electron. ...
... on a string. Higher energy states are then similar to harmonics of the fundamental frequency. 2. The electrons are never in a single point location, although the probability of interacting with the electron at a single point can be found from the wave function of the electron. ...
Rutherford backscattering spectrometry
Rutherford backscattering spectrometry (RBS) is an analytical technique used in materials science. Sometimes referred to as high-energy ion scattering (HEIS) spectrometry, RBS is used to determine the structure and composition of materials by measuring the backscattering of a beam of high energy ions (typically protons or alpha particles) impinging on a sample.




![L 33 Modern Physics [1] Modern Physics](http://s1.studyres.com/store/data/003217156_1-265c5a519e2bca3f33717b4abd842898-300x300.png)

![L 34 Modern Physics [1]](http://s1.studyres.com/store/data/008622077_1-047a8df5b8f51427a7d951942e25e95f-300x300.png)


![L 35 Modern Physics [1] - University of Iowa Physics](http://s1.studyres.com/store/data/001147028_1-f00aa7577568b42bc32948cbade9023a-300x300.png)