* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Document
Quantum electrodynamics wikipedia , lookup
Chemical bond wikipedia , lookup
Bremsstrahlung wikipedia , lookup
Molecular Hamiltonian wikipedia , lookup
Hydrogen atom wikipedia , lookup
Tight binding wikipedia , lookup
Wave–particle duality wikipedia , lookup
Atomic orbital wikipedia , lookup
Auger electron spectroscopy wikipedia , lookup
Theoretical and experimental justification for the Schrödinger equation wikipedia , lookup
Rutherford backscattering spectrometry wikipedia , lookup
X-ray photoelectron spectroscopy wikipedia , lookup
X-ray fluorescence wikipedia , lookup
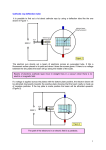
Physikalisches Anfaengerpraktikum Franck-Hertz-Versuch Ausarbeitung von Constantin Tomaras & David Weisgerber (Gruppe 10) Montag, 24. Oktober 2005 eMail: [email protected] 1 (1) Introduction To deliver the proof of a theory by "Townsend“, the German physicists Franck and Hertz have experimented in Berlin since 1911 to examine collisions between electrons and gas atoms. In their original work, they concluded that the measured 4,9eV must be the ionization energy of the mercury gas. After Nils Bohr commented this theory it was obvious that the electrons collided with the mercury inelastic and it was possible to establish the link between the energy loss of the electrons and the light emission of the gas which could be watched. The illumination of the mercury came from electrons with a kinetic energy of more than 4,9eV and additionally a monochromatic radiation of 253,7nm could be measured. This agreed to Einstein's formula: E=h⋅f E kin=e⋅U ⇒ h⋅ f =e⋅U In the further process of the experiment, several other energy states of the mercury gas were found. (2) Experimental Overview In a tube containing mercury gas (in order to vaporize the mercury, the tube was heated by a surrounding oven at approximately 460K) electrons were accelerated by variable potential between a heated cathode and an anode grid G. After passing the grid the electrons are slowed down by a reverse potential until they hit the electron collector A. The current of the electron collector is measured as a function against the acceleration potential: 2 The graph of the function current against acceleration potential is explained by the process of inelastic collisions between the electrons and the heated mercury gas atoms inside the tube. By being accelerated between the cathode and the anode grid the electrons collect enough energy to energize a mercury gas atom which will afterwards lose its energy by sending out a photon with the same energy as the electron lost by the inelastic collision. Due to the (by Bohr's theory not answered) fact that atoms can only be energized by discrete energies, the first possibility of the electrons to energize the mercury is at a kinetic energy of 4.9eV. If the electrons are not accelerated any more after the collision, they are not able to reach the electron collector's cathode and the current at this cathode is decreased. By rising the acceleration potential, it is possible for the electrons to do several inelastic collisions which means that a local maximum of current at the electron collector can be expected at voltages of n*4.9V. In order to gain a better understanding of the whole physical effect this theoretical overview shall be given: (3) Theoretical Overview To give a theoretical overview of the underlying atom model we start with the well-known model of Rutherford: It says that the atom consists of a positive charged core and several negative charged electrons which are flying around the core on circular paths. The Bohr model says that these electrons can only be found on discrete paths with discrete energy values. It does not declare why the electrons do not send out electromagnetic radiation according to Maxwell's equitations. This phenomena can only be declared with the model of quantum physics. To describe the effects we found in this experiment we do not need an insight view into the model of quantum physics (as this would also go to far for this work) but we can examine the effects we found with the postulates of Bohr: (i) The angular momentum only assumes discrete values according to L=r⋅m⋅v= n⋅h ⋅ 2 h⋅ 2 n=1,2,3 , ... On these discrete paths the electrons can be found. n is also known as the quantum number. (ii) Atoms can only be energized by discrete energy values: If an electron is changing its course radius (where only discrete course radial are allowed) it emits a photon with a frequency according to the lower potential and kinetic energy: f= E a −E b h 3 According to this formula, atoms absorb energy by increasing potential and kinetic energy of electrons. As a result of the first postulate there can only be discrete energy values be absorbed or emitted. However, further explanations of the effects watched in this experiments can only be made by using the model of quantum physics. At least with this underlying model, a theoretical value of 4.9eV for the energizing energy of mercury could be calculated. (4) Experiment (i) Assembly A scheme of the electric circuit of our experimental tube can be found in chapter 2. In both experiments, with mercury gas and neon the wiring of the tubes and the measuring devices was the same. In the experiment with mercury gas, the tube was heated by approximately 460K to ensure that the mercury is in the state of gas. The electron collector's cathode is connected to a small amplifier. The signal of this amplifier is connected to the vertical input of our oscilloscope. Between cathode and anode grid was put on a varying voltage of 0V to 45V. This voltage was also connected to the horizontal input of our oscilloscope. In the experiment with the neon tube this voltage could vary between 0V and approximately 80V. (ii) Measurements with the mercury gas By calibrating the heating of the cathode, the counter voltage between anode grid and electron collector's cathode and adjusting the amplifier and the electron beam of our oscilloscope we tried to find a graph with sharp maximums. In order to achieve this the acceleration voltage was put to alternating voltage between 0V and 45V. An imprecise draw of this graph can be found in our protocol block. This part of the experiment was only thought to find good values for the counter voltage and the heating voltage of the cathode so this drawing has no scientific relevance. In order to gain the exact positions of the maximums of the electron collector's current we used these values for counter voltage, temperature and maximum acceleration voltage: U counter =2,08V T =451K ... 464K U acceleration , max =45V In order to find a the maximums of the electron collector's current very precise we connected an analogue voltage measurement device to amplifiers output and we used a digital multimeter connected to the cathode and the anode grid to get precise values of the acceleration voltage. We measured these values: Maximum (U) Distance between maxima 16.1V 21.1V 5V 26.2V 5.1V 31.2V 5V 4 36.2V 5V 42.1V 6.1V That means that we get as an average of the distance of the maxima: n 1 m= ∑ U x n x=1 26.2V maverage = =5.2V 5 We made these calculations for the systematic an the statistic error: statistic error : 1 m1 = ⋅s n s= n 1 ⋅∑ U x −m2 n−1 x=1 m1=0.21V systematic error : m2=0.5V total error : m=m1m2=0.71V So our result for the average of the distance of the maxima with error is: maverage =5.2V±0.7V With this result we are able to calculate the wavelength of the photons which are emitted: h⋅c =237 nm±17 nm E=5.2eV±0.7eV= Our results of a distance between two maxima and the wavelength of the emitted photon are with values of maverage =5.2V±0.7V and =237 nm±17 nm within the expected theoretical values of 4.9eV and 253.7nm. (iii) Measurements with the neon tube The second tube we examined was filled with neon which we did not need to heat because it is in the state of gas at room temperature. The process of the experiment was more or less exactly the same like the process of the mercury gas tube. A little difference to the mercury tube was the higher maximum voltage of the alternating voltage of 80V. With this voltage we tried to find a graph with sharp maximums and got this value for the counter voltage between anode grid and electron collector's cathode: U counter =4.8V 5 Exactly like we did with the mercury tube we found these values for the maxima of the neon tube: Maximum (U) Distance between maxima 18.7V 36.8V 18.1V 55.6V 18.8V 77.8V 22.2V That means that we get as an average of the distance of the maxima: n m= 1 ∑U n x=1 x 1 maverage = ⋅18.1V 18.8V22.2V=19.7V 3 We made these calculations for the systematic an the statistic error: statistic error : 1 m1 = ⋅s n s= n 1 ⋅∑ U −m2 n−1 x=1 x m1=1.27V systematic error : m2=1.0V total error : m=m1m2=2.27V So our result for the average of the distance of the maxima with error is: maverage =19.7V±2.3V With this result we are able to calculate the wavelength of the photons which are emitted: h⋅c =629 nm±54 nm E=19.7eV±2.3eV= The wavelength we found here does not exist. This 19eV transition consists of two different transitions. The one is a 2eV transition and the other is a 17eV transition. So there are two different wavelengths emitted. 6 (iv) Wavelength measurement with a spectrometer In order to measure the wavelengths emitted by the neon tube we use a classic optical device, the pocket spectrometer. The scale of the spectrometer was calibrated using a neon lamp and afterwards we measured the wavelengths of the photons emitted by our neon tube with a precision of ± 5nm. We got the following values: orange =620 nm±5 nm red =660 nm±5 nm green =655 nm±5 nm (5) Questions (i) Explain the terms elastic collision and inelastic collision An elastic collision is a collision in which the total kinetic energy of the colliding bodies after collision is equal to their total kinetic energy before collision. Elastic collisions occur only if there is no conversion of kinetic energy into other forms, as in the collision of atoms. Inelastic collision is a collision in which some of the kinetic energy of the colliding bodies is converted into internal energy in one body so that kinetic energy is not conserved. In collisions of macroscopic bodies some kinetic energy is turned into vibrational energy of the atoms, causing a heating effect (ii) Why is an electron at energies below 4.9 eV only able to perform elastic collisions? Electrons with energies below 4.9 eV are not able to perform inelastic collisions because they do not have enough energy to energize the atom it is colliding with. In addition, the mass of the atom is much bigger than the mass of a single electron so the collision can be compared to a ball hitting a wall. (iii) Why is the energy an electron can transfer to an atom low in elastic collisions? Compared to an atom the mass of an electron is very low. The mass of a mercury atom is 200.59 u while the mass of an electron is only 5.48580⋅10−4 u . (iv) How does an atom excited by an inelastic collision dispose itself of the acquired energy? The energy is emitted in form of a photon. (v) What is the difference between the excitation of an atom by electrons and by light quanta? While electrons can loose just portions of their kinetic energy, light quanta are fully absorbed by atoms. As a result of this, light quanta must match the difference between two discrete energy values of an atom in order to energize it. If it does not match it will not be absorbed. (vi) Why is it necessary to apply a deceleration voltage between collector electrode and anode grid? 7 If no deceleration voltage is applied between electron collector's cathode and the anode grid the maxima might not be very sharp. In addition, if electrons have just a low amount of kinetic energy they will hit the electron collector. (vii) Compare the functionality of a Franck-Hertz tube with that of a fluorescent lamp and try to understand this lamp with the help of the schematic sketch. Why are these lamps called fluorescent lamps? [cf. Bergmann-Schäfer, Lehrbuch der Experimentalphysik, vol. III (Optik)] The common fluorescent tube relies on fluorescence. Inside the glass tube is a partial vacuum and a small amount of mercury. An electric discharge in the tube causes the mercury atoms to emit light. The emitted light is in the ultraviolet range and is invisible, and also harmful to living organisms, so the tube is lined with a coating of a fluorescent material, called the phosphor, which absorbs the UV and re-emits visible light. (viii) What is the difference to an x-ray tube? In a x-ray tube the high energy photons are emitted when the electrons hit the anode with high kinetic energy while in fluorescent tubes the photons are emitted when the electrons hit gas atoms. 8