Atomic Spectra
... ΔS = 0 stems from the fact that the photon, which has a spin of 1, does not affect the spin directly. The rules about ΔL and Δl express the fact that the orbital angular momentum of an individual electron must change, but whether or not this results in an overall change of orbital momentum depends o ...
... ΔS = 0 stems from the fact that the photon, which has a spin of 1, does not affect the spin directly. The rules about ΔL and Δl express the fact that the orbital angular momentum of an individual electron must change, but whether or not this results in an overall change of orbital momentum depends o ...
Spectrophotometry Chapter 18
... • Not all energies exist, only certain allowed energy levels. • Electrons with more energy are able to get farther away from the nucleus and its + charges. • Therefore, electrons in higher energy levels spend more time farther away from the nucleus. • The higher energy levels are larger so they can ...
... • Not all energies exist, only certain allowed energy levels. • Electrons with more energy are able to get farther away from the nucleus and its + charges. • Therefore, electrons in higher energy levels spend more time farther away from the nucleus. • The higher energy levels are larger so they can ...
Chem 152 Chapter 4
... – hydrogen and oxygen react to form water. – 2H2 + O2 2H2O – Reactants on left; products on right. – Heat represented by . Conservation of Mass – No change is observed in the total mass of the substances involved in a chemical ...
... – hydrogen and oxygen react to form water. – 2H2 + O2 2H2O – Reactants on left; products on right. – Heat represented by . Conservation of Mass – No change is observed in the total mass of the substances involved in a chemical ...
Chemistry Semester Test Study Guide Chapters
... What state of matter has a definite volume and takes the shape of its container? Which state of matter takes both the shape and volume of its container? In a chemical reaction, what are the reactants and what are the products? If the total mass of the reactants in a chemical reaction is 60 g, what i ...
... What state of matter has a definite volume and takes the shape of its container? Which state of matter takes both the shape and volume of its container? In a chemical reaction, what are the reactants and what are the products? If the total mass of the reactants in a chemical reaction is 60 g, what i ...
Unit_Chemistry_2_Ionic_Substances_and_Electrolysis
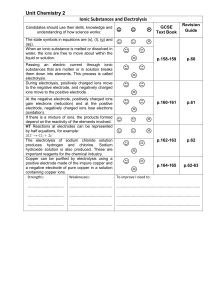
... Ionic Substances and Electrolysis Candidates should use their skills, knowledge and understanding of how science works: ...
... Ionic Substances and Electrolysis Candidates should use their skills, knowledge and understanding of how science works: ...
Unit 4 Evolution
... Let’s review yesterday’s activities and begin a couple of review activities over energy and properties of matter. ...
... Let’s review yesterday’s activities and begin a couple of review activities over energy and properties of matter. ...
AP Chemistry Summer Packet More Chapter Two and Chapter
... e. None of the above 76. When a substance that has a positive charge is brought near a substance that has a negative charge, a force of attraction occurs between them. WHen two substances with the same sign of charge are brought near each other, a repulsive force occurs. These forces are electrostat ...
... e. None of the above 76. When a substance that has a positive charge is brought near a substance that has a negative charge, a force of attraction occurs between them. WHen two substances with the same sign of charge are brought near each other, a repulsive force occurs. These forces are electrostat ...
CHEMISTRY VOCABULARY
... An ATOM is the smallest part of an element. ELEMENTS cannot be broken down by either physical or chemical methods. In PHYSICAL processes no new substance is made. In CHEMICAL processes something new is made. ATOMS have a nucleus, which contains protons and neutrons, Electrons are arranged around the ...
... An ATOM is the smallest part of an element. ELEMENTS cannot be broken down by either physical or chemical methods. In PHYSICAL processes no new substance is made. In CHEMICAL processes something new is made. ATOMS have a nucleus, which contains protons and neutrons, Electrons are arranged around the ...
Unit 1 VCE Physics: Sample Timeline, 2004
... Computer simulation of a reactor including a “meltdown”. Class debate after library research Essay. ...
... Computer simulation of a reactor including a “meltdown”. Class debate after library research Essay. ...
Honors Chemistry First Marking Period Review Sheet
... I can determine the number of electrons in a particular shell ( 2n2 ), subshell (see table above), and orbital (2 electrons). I can apply the Heisenberg uncertainty principle: It is impossible to determine both the position and the momentum of an electron at the same time. For this reason, only the ...
... I can determine the number of electrons in a particular shell ( 2n2 ), subshell (see table above), and orbital (2 electrons). I can apply the Heisenberg uncertainty principle: It is impossible to determine both the position and the momentum of an electron at the same time. For this reason, only the ...
Prerequisite Knowledge for Chemistry
... Compounds are represented by chemical formulas consisting of element symbols and subscripts. For instance, water’s chemical formula is H2O. The “H” stands for hydrogen and the “O” stands for oxygen. The subscripts come after the element to which they refer. Subscripts state the number of atoms of th ...
... Compounds are represented by chemical formulas consisting of element symbols and subscripts. For instance, water’s chemical formula is H2O. The “H” stands for hydrogen and the “O” stands for oxygen. The subscripts come after the element to which they refer. Subscripts state the number of atoms of th ...
Study Guide: Chapter 4 - the Arrangement of Electrons in Atoms
... Study Guide: Chapter 4 - the Arrangement of Electrons in Atoms 1. Understand the relationship between a light wave’s frequency and wavelength; Know how to calculate wavelength given frequency and frequency given wavelength (MEMORIZE FORMULA) – work a few practice problems 2. Understand the relations ...
... Study Guide: Chapter 4 - the Arrangement of Electrons in Atoms 1. Understand the relationship between a light wave’s frequency and wavelength; Know how to calculate wavelength given frequency and frequency given wavelength (MEMORIZE FORMULA) – work a few practice problems 2. Understand the relations ...
UV-Vis Absorption Spectroscopy
... Effect of Scattered Radiation at Wavelength Extremes of an Instrument Wavelength extremes of an instrument are dependent on type of source, detector and optical components used in the manufacture of the instrument. Outside the working range of the instrument, it is not possible to use it for accura ...
... Effect of Scattered Radiation at Wavelength Extremes of an Instrument Wavelength extremes of an instrument are dependent on type of source, detector and optical components used in the manufacture of the instrument. Outside the working range of the instrument, it is not possible to use it for accura ...
Chapter 7
... behavior (an example…photoelectric effect) Particles (the electron) has wave like behavior (example: the emission spectrum) Our goals in the first part of this chapter is To describe light and particles in terms of energy and wavelength, apply DeBroglie’s relationship and the photoelectric effect To ...
... behavior (an example…photoelectric effect) Particles (the electron) has wave like behavior (example: the emission spectrum) Our goals in the first part of this chapter is To describe light and particles in terms of energy and wavelength, apply DeBroglie’s relationship and the photoelectric effect To ...
ก F ก F U234 92
... 32ºC. Pure toluene has a vapor pressure of 41 torr at 32ºC. (Assume ideal behavior.) 4. The boiling point of ethanol (C2H5OH) is 78.5ºC. What is the boiling point of a solution of 6.4 g of vanillin (MW = 152.14 g/mol) in 50.0 g of ethanol (Kb of ethanol = 1.22ºC/m)? 5. The U.S. Food and Drug Adminis ...
... 32ºC. Pure toluene has a vapor pressure of 41 torr at 32ºC. (Assume ideal behavior.) 4. The boiling point of ethanol (C2H5OH) is 78.5ºC. What is the boiling point of a solution of 6.4 g of vanillin (MW = 152.14 g/mol) in 50.0 g of ethanol (Kb of ethanol = 1.22ºC/m)? 5. The U.S. Food and Drug Adminis ...
Chemical Equations and Tests for anions
... Law of Conservation of Matter In any chemical reaction matter is neither created nor destroyed but merely changes from one form to another If there is a particular number of atoms at the start of a reaction then there must be the same number of atoms at the end of the reaction ...
... Law of Conservation of Matter In any chemical reaction matter is neither created nor destroyed but merely changes from one form to another If there is a particular number of atoms at the start of a reaction then there must be the same number of atoms at the end of the reaction ...
The Chemical Basis of Life
... – Elements are listed in order of their atomic numbers – Elements are designated by standard one or twoletter abbreviations – Elements in the same vertical column often have very similar chemical bonding properties ...
... – Elements are listed in order of their atomic numbers – Elements are designated by standard one or twoletter abbreviations – Elements in the same vertical column often have very similar chemical bonding properties ...
ME 533 Lecture 6 Pla..
... • To designate whether the wave function is symmetric or antisymmetric WRT reflection at any plane including the internuclear axis,- in the case of Σ-states (so when Λ=0), the right hand superscripts “+” and “-” are used. • ,Further, to designate whether the wave function is symmetric or antisymmetr ...
... • To designate whether the wave function is symmetric or antisymmetric WRT reflection at any plane including the internuclear axis,- in the case of Σ-states (so when Λ=0), the right hand superscripts “+” and “-” are used. • ,Further, to designate whether the wave function is symmetric or antisymmetr ...
Unit 01 Qual Chem
... Chemical Change = a change in which one or more substances are converted into substances with different chemical properties ...
... Chemical Change = a change in which one or more substances are converted into substances with different chemical properties ...
Chemistry Curriculum Guide
... Using a periodic chart, determine the atomic number, atomic mass, the number of protons, the number of electrons, and the number of neutrons of any neutral atom of a particular element. ISOTOPES, HALF LIVES, AND RADIOACTIVE DECAY ...
... Using a periodic chart, determine the atomic number, atomic mass, the number of protons, the number of electrons, and the number of neutrons of any neutral atom of a particular element. ISOTOPES, HALF LIVES, AND RADIOACTIVE DECAY ...
Lecture 4 (October 1, 2007): Quantum Statistical Mechanics
... electrons as independent, uncorrelated states. Recall that a) the overall electron wf must be antisymmetric under exchange of electrons (since they are fermions), which means that the product of space wf and spin wf for the 2-electron system must be odd. We know that the spin wf can be either symmet ...
... electrons as independent, uncorrelated states. Recall that a) the overall electron wf must be antisymmetric under exchange of electrons (since they are fermions), which means that the product of space wf and spin wf for the 2-electron system must be odd. We know that the spin wf can be either symmet ...
Rutherford backscattering spectrometry
Rutherford backscattering spectrometry (RBS) is an analytical technique used in materials science. Sometimes referred to as high-energy ion scattering (HEIS) spectrometry, RBS is used to determine the structure and composition of materials by measuring the backscattering of a beam of high energy ions (typically protons or alpha particles) impinging on a sample.