* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Phonons II
Ising model wikipedia , lookup
Planck's law wikipedia , lookup
Relativistic quantum mechanics wikipedia , lookup
Hydrogen atom wikipedia , lookup
Molecular Hamiltonian wikipedia , lookup
Lattice Boltzmann methods wikipedia , lookup
Particle in a box wikipedia , lookup
Franck–Condon principle wikipedia , lookup
Electron scattering wikipedia , lookup
X-ray fluorescence wikipedia , lookup
Elementary particle wikipedia , lookup
Matter wave wikipedia , lookup
Tight binding wikipedia , lookup
Bohr–Einstein debates wikipedia , lookup
Rutherford backscattering spectrometry wikipedia , lookup
Wave–particle duality wikipedia , lookup
Theoretical and experimental justification for the Schrödinger equation wikipedia , lookup
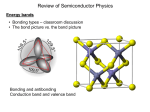
Lattice Vibrations Part II Solid State Physics 355 Three Dimensions For each mode in a given propagation direction, the dispersion relation yields acoustic and optical branches: • Acoustic • Longitudinal (LA) • Transverse (TA) • Optical • Longitudinal (LO) • Transverse (TO) If there are p atoms in the primitive cell, there are 3p branches in the dispersion relation: 3 acoustic and 3p -3 optical. NaCl – two atoms per primitive cell 6 branches: 1 LA 1 LO 2 TA 2 TO Counting This enumeration follows from counting the number of degrees of freedom of the atoms. For p atoms in N primitive cells, there are pN atoms. Each atom has 3 degrees of freedom, one for each of the 3 directions x, y, and z. This gives 3Np degrees of freedom for the crystal. q = ±/a q. q Three Dimensions un ue i ( q r t ) Al Ge Quantization of Elastic Waves The energy of an elastic mode of angular frequency is E n n 12 It is quantized, in the form of phonons, similar to the quantization of light, as both are derived from a discrete harmonic oscillator model. Elastic waves in crystals are made up of phonons. Thermal vibrations are thermally excited phonons. Phonon Momentum A phonon with a wavevector q will interact with particles, like neutrons, photons, electrons, as if it had a momentum (the crystal momentum) p q • Be careful! Phonons do not carry momentum like photons do. They can interact with particles as if they have a momentum. For example, a neutron can hit a crystal and start a wave by transferring momentum to the lattice. • However, this momentum is transferred to the lattice as a whole. The atoms themselves are not being translated permanently from their equilibrium positions. • The only exception occurs when q = 0, where the whole lattice translates. This, of course, does carry momentum. Phonon Momentum For example, in a hydrogen molecule the internuclear vibrational coordinate r1 r2 is a relative coordinate and doesn’t have linear momentum. The center of mass coordinate ½(r1 r2 ) corresponds to the uniform mode q = 0 and can have linear momentum. H2 electron r R r R Proton A Proton B r1 r2 O Phonon Momentum p q Earlier, we saw that the elastic scattering of x-rays from the lattice is governed by the rule: k k G IfIfthe is inelastic, with a creation of a thephoton photonscattering is absorbed, then phonon of wavevector q, then kk Gq q kkG Phonon Scattering (Normal Process) q1 q3 = q1 + q2 q2 q3 = q1 + q2 or q3 = q1 + q2 + G Measuring Phonons k q k G reciprocal lattice vector scattered neutron phonon wavevector (+ for phonon created, for phonon absorbed) Stokes or anti-Stokes Process incident neutron Measuring Phonons q Measuring Phonons Measuring Phonons Other Techniques • Inelastic X-ray Spectroscopy • Raman Spectroscopy (IR, near IR, and visible light) • Microwave Ultrasonics Heat Capacity You may remember from your study of thermal physics that the specific heat is the amount of energy per unit mass required to raise the temperature by one degree Celsius. Q = mcT Thermodynamic models give us this definition: U CV T V Cv = yT+T3 electrons phonons Heat Capacity Equipartition Theorem: The internal energy of a system of N particles is 3 2 Nk BT Monatomic particles have only 3 translational degrees of freedom. They possess no rotational or vibrational degrees of freedom. Thus the average energy per degree of freedom is 1 2 Nk BT It turns out that this is a general result. The mean energy is spread equally over all degrees of freedom, hence the terminology – equipartition. Heat Capacity Heat Capacity Answer: You need to use quantum statistics to describe this properly. Bosons and Fermions Bosons: particles that can be in the same energy state (e.g. photons, phonons) Fermions: particles that cannot be in the same energy level (e.g. electrons) Planck Distribution Max Planck – first to come up with the idea of quantum energy worked to explain blackbody radiation empty cavity at temperature T, with which the photons are in equilibrium Planck Distribution Einstein Model 1907-Einstein developed first reasonably satisfactory theory of specific heat capacity for a solid assumed a crystal lattice structure comprising N atoms that are treated as an assembly of 3N one-dimensional oscillators approximated all atoms vibrating at the same frequency (unrealistic, but makes things easier) Planck Distribution number of phonons in energy level n total number of phonons all possible energy levels 0, 1, 2, etc. Planck Distribution n n nhf Fraction of Phonons at energy n Nn N 0 n e n / kT e 0 n / kT e n / kT e 0 n / kT small as n gets large a constant Planck Distribution average occupied energy level 1 1 1 n / k BT n x e e / k BT 1 e 1 e 1 x n 0 n 0 n 0 n x n n 1 nx x nx xx x x nn00 x 1 x 1 x 2 n 0 nn Einstein Model average energy per oscillator We have 3N such oscillators, so the total energy is Einstein Model k BT dv and dT k BT 2 let v Einstein Model How did Einstein do? T Einstein Model How did Einstein do? T 0K Einstein Model The Einstein model failed to identically match the behavior of real solids, but it showed the way. In real solids, the lattice can vibrate at more than one frequency at a time. Answer: the Debye Model