* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Multielectron Atoms * The Independent Particle Approximation
Molecular Hamiltonian wikipedia , lookup
Franck–Condon principle wikipedia , lookup
Atomic orbital wikipedia , lookup
Hydrogen atom wikipedia , lookup
Electron configuration wikipedia , lookup
X-ray photoelectron spectroscopy wikipedia , lookup
Bohr–Einstein debates wikipedia , lookup
Matter wave wikipedia , lookup
X-ray fluorescence wikipedia , lookup
Rutherford backscattering spectrometry wikipedia , lookup
Wave–particle duality wikipedia , lookup
Tight binding wikipedia , lookup
Atomic theory wikipedia , lookup
Theoretical and experimental justification for the Schrödinger equation wikipedia , lookup
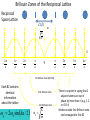
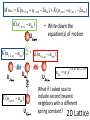
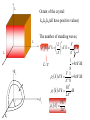
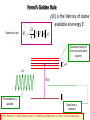
Main Topics from Chapters 3-5 Due to time, not all topics will be on test. Some problems ask to discuss the meaning or implication. Lattice Dynamics (Monatomic, Diatomic, Mass Defect, 2D Lattices) Strain (compliance, reduced notation, tensors) Harmonic Oscillator (Destruction/Creation, Hamiltonian & Number Operators, Expectation Values) Energy Density and Heat Capacity (phonons, electrons and photons) Quasiparticle Interactions (e-e, e-phonon, e-photon, defect interations) Electrical and Thermal Conductivity Lattice Vibrations When a wave propagates along one direction, 1D problem. Use harmonic oscillator approx., meaning amplitude vibration small. The vibrations take the form of collective modes which propagate. Phonons are quanta of lattice vibrations. Longitudinal Waves Transverse Waves Monatomic Linear Chain The force on the nth atom; a a •The force to the right; K (u n1 u n ) •The force to the left; K (u n u n1 ) Un-1 Un Un+1 The total force = Force to the right – Force to the left Thus, Newton’s equation for the nth atom is mun K un un1 K un un1 .. m u n K (un 1 2un un 1 ) Eqn’s of motion of all atoms are of this form, only.. the value of ‘n’ varies 0 un A exp i kxn t u n 2un Brillouin Zones of the Reciprocal Lattice Reciprocal Space Lattice: 2/a 4K M k 4 a 3 a 2 a 0 a a 2 a 3 a 4 a 1st Brillouin Zone (BZ=WS) Each BZ contains identical information about the lattice q 20 sin ka / 2 2nd Brillouin Zone 3rd Brillouin Zone 0 K m There is no point in saying that 2 adjacent atoms are out of phase by more than (e.g., 1.2 =-0.8 ) Modes outside first Brillouin zone can be mapped to first BZ Are These Waves Longitudinal or Transverse? Four examples of standing waves in a linear crystal corresponding to q = 1, 2, 4, and N. q is maximum when alternating atoms are vibrating in opposite directions. A portion from a very long crystal is shown. Fig 4.43 From Principles of Electronic Materials and Devices, Third Edition, S.O. Kasap (© McGraw-Hill, 2005) Diatomic Chain(2 atoms in primitive basis) 2 different types of atoms of masses m1 and m2 are connected by identical springs (n-2) (n-1) K (n) K m1 m2 (n+1) K K m1 (n+2) m m2 a) a b) Un-2 Un-1 Un Un+1 Un+2 Since a is the repeat distance, the nearest neighbors separations is a/2 Two equations of motion must be written; One for mass m1, and One for mass m2. m1un1 K 2un1 un 2 un 1, 2 m2un 2 K 2un 2 un1 un 1,1 2 2 1 2 2 2 1 4 2 2 2 2 1 2 2 sin qa / 2 2 1/ 2 • As there are two values of ω for each value of k, the dispersion relation is said to have two branches A Optical Branch Upper branch is due to the positive sign of the root. B C Acoustical Branch –л/a 0 л/a 2л/a k Negative sign: k for small k. Dispersionfree propagation of sound waves • At C, M oscillates and m is at rest. • At B, m oscillates and M is at rest. • This result remains valid for a chain containing an arbitrary number of atoms per unit cell. A when the two atoms oscillate in antiphase Number and Type of Branches • Every crystal has 3 acoustic branches, 1 longitudinal and 2 transverse • Every additional atom in the primitive basis contributes 3 further optical branches (again 2 transverse and 1 longitudinal) .. M u lm K (ul 1,m ul 1,m 2ulm ) K (ul ,m 1 ul ,m 1 2ulm ) K (ul ,m 1 ulm ) Ul,m+1 K (ul 1,m ulm ) Ul-1,m K Ulm K (ul ,m 1 ulm ) Ul,m-1 • Write down the equation(s) of motion K (ul 1,m ulm ) Ul+1,m ulm uo e i ( lk x a mk y a t ) What if I asked you to include second nearest neighbors with a different spring constant? 2D Lattice .. M u lm K (ul 1,m ul 1,m 2ulm ) K (ul ,m 1 ul ,m 1 2ulm ) Ul,m+1 2K (2 cos k x a cos k y a ) M 2 C Ul-1,m Ulm Ul,m-1 2D Lattice Ul+1,m Similar to the electronic bands on the test, plot w vs k for the [10] and [11] directions. Identify the values of at k=0 and at the edges. Specific Heat or Heat Capacity The heat energy required to raise the temperature a certain amount The thermal energy is the dominant contribution to the heat capacity in most solids. In non-magnetic insulators, it is the only contribution. Classical Picture of Heat Capacity When the solid is heated, the atoms vibrate around their sites like a set of harmonic oscillators. Therefore, the average energy per atom, regarded as a 3D oscillator, is 3kT, and consequently the energy per mole is = 3NkBT 3RT d Cv dT Dulong-Petit law: states that specific heat of any solid is independent of temperature and the same result (3R~6cal/K-mole) for all materials! Thermal Energy & Heat Capacity Average energy of a harmonic oscillator and hence of a lattice mode at temperature T Einstein Model n Pn n _ Pn n in The probability of the oscillator being this level as given by the Boltzman factor Energy of oscillator exp( n / kBT ) 1 1 n exp n / k T B _ 2 2 n 0 1 exp n / k T B 2 n 0 _ 1 2 e / k BT 1 n n 2 1 kBT Mean energy of a harmonic oscillator High Temperature Limit 1 2 << kBT T Low Temperature Limit kBT exponential 1 term gets kBT 2 e 1 bigger _ _ 1 2 Zero Point Energy is independent of frequency of oscillation. 2 x e x 1 x .......... 2! e 1 k BT _ 1 2 1 1 k BT _ 1 k BT 2 This is a classical limit because the energy steps are now small compared with thermal/vibrational energy k BT _ kBT Heat Capacity C (Einstein) Heat capacity found by differentiating average phonon energy d Cv dT Cv kB k BT 2 e k 1 e 2 k BT kBT e Cv k B T e T 1 Area = 2 where T B kB 2 2 k The difference between classical and Einstein models comes from zero point energy. T(K) The Einstein model near T= 0 did not agree with experiment, but was better than classical model. Taking into account the distribution of vibration frequencies in a solid this discrepancy can be accounted for. Points:Experiment Curve: Einstein Prediction Debye approximation has two main steps 1. Approx. dispersion relation of any branch by a linear extrapolation 2. Ensure correct number of modes by imposing a cut-off frequency D, above which there are no modes. The cut-off freqency is chosen to make the total number of lattice modes correct. Since there are 3N lattice vibration modes in a crystal having N atoms, we choose D so that: Einstein approximation to the dispersion Debye approximation to the dispersion V vk 6 2 D g ( )d 3N 0 ( 1 2 )D3 3 N 3 3 vL vT V 1 2 D 2 ( 3 3 ) d 3 N 2 2 vL vT 0 V 1 2 3N 9N ( ) 3 2 2 vL3 vT3 D3 D3 g ( ) 9N D3 2 g ( ) / 2 Density of states (DOS) per unit frequency range g() • The number of modes/states with frequencies and +d will be g()d. # modes with wavenumber from k to k+dk= dn S (k )dk g ( )d dk for 1D monoatomic lattice d d K ka 2a 2 sin dk m 2 2 1 g () S (k ) K ka a cos m 2 g ( ) S (k ) K ka cos m 2 sin 2 x cos2 x 1 cos x 1 sin 2 x 1 L 2 g ( ) 2 a max 2 Debye Model adjusts Einstein Model The energy of lattice vibrations will then be found by integrating the energy of single oscillator over the distribution of vibration frequencies. Thus 1 2 e 0 / kT g d 1 2N Mean energy of a harmonic oscillator 2 max for 1D 2 1/ 2 It would be better to find 3D DOS in order to compare the results with experiment. 3D Example: The number of allowed states per unit energy range for free electron? • Each k state represents two possible electron states, one for spin up, the other is spin down. g ( E )dE 2 g (k )dk 2 2 k E 2m 2 dE k dk m V m 2mE dk kk g ( E ) 2 g ( k2) 2 2 2 dEk dk g ( E ) 2 g (k ) dE k 2mE g (E) 2 V 2 2 3/ 2 1/ 2 (2 m ) E 3 Vk 2 g (k )dk 2 dk , 2 L Octant of the crystal: kx,ky,kz(all have positive values) The number of standing waves; L 3 V 3 L 3 s k d k d k 3 d k 1 4 k 2 dk L / 8 V 1 3 s k d k 3 4 k 2 dk 8 2 Vk s k d 3k 2 dk 2 ky Vk 2 S k 2 2 3 L kz dk k kx The Heat Capacity of a Cold Fermi Gas (Metal) U T g f , T d 0 Close to EF, we can ignore the variation in the density of states: g() g(EF). By heating up a metal (kBT << EF), we take a group of electrons at the energy - (with respect to EF), and “lift them up” to . The number of electrons in this group g(EF)f()d and each electron increased its energy by 2 : kBT U U 0 2 g EF f , T d 0 dU T 2 Ce g EF k B2T dT 3 3 n g E F 2 EF Ce 2 2 Nk B k BT EF The small heat capacity of metals is a direct consequence of the Pauli principle. Most of the electrons cannot change their energy. Otherwise metals would have infinite conductivity On average, I go about seconds between collisions electron Bam! phonon Random Collisions with phonons and impurities The thermal vibration of the lattice (phonons) will prevent the atoms from ever all being on their correct sites at the same time. The presence of impurity atoms and other point defects will upset the lattice periodicity Electrons colliding with phonons (T > 0) Electrons colliding with impurities ph T 0 imp is independent of T Fermi’s Golden Rule Transition rate: 2 W fi (E) is the ‘density of states available at energy E’. f Hp i 2 f Quantum levels of the non-perturbed system f ,f Perturbation is applied i Transition is induced • See Fermi‘s Golden Rule paper in Additional Material on the course homepage Absorption When the ground state finds itself in the presence of a photon of the appropriate frequency, the perturbing field can induce the necessary oscillations, causing the mix to occur. This leads to the promotion of the system to the upper energy state and the annihilation of the photon. This process is stimulated absorption (or simply absorption). f - degeneracy of state f f fi Einstein pointed out that the Fermi Golden Rule correctly describes the absorption process. fi Ni i Quantum Oscillator Atoms still have energy at T=0. What is <x2> for the ground state of the quantum harmonic oscillator? x (a a ) 2m 0 (a a ) 2 0 2m 0 a 2 0 0 a 2 0 0 a a 0 0 a a 0 2m x2 0 0 0 1 2m 2m ⇒ (1D Case) For 3D quantum oscillator, the result is multiplied by 3: x 2 3 2m G 2 I I 0 exp 2M • These quantized normal modes of vibration are called PHONONS • PHONONS are massless quantum mechanical particles which have no classical analogue. – They behave like particles in momentum space or k space. • Phonons are one example of many like this in many different areas of physics. Such quantum mechanical particles are often called “Quasiparticles” Examples of other Quasiparticles: Photons: Quantized Normal Modes of electromagnetic waves. Magnons: Quantized Normal Modes of magnetic excitations in magnetic solids Excitons: Quantized Normal Modes of electron-hole pairs Phonon spectroscopy = Conditions for: inelastic scattering Constraints: Conservation laws of Momentum Energy In all interactions involving phonons, energy must be conserved and crystal momentum must be conserved to within a reciprocal lattice vector. Deformation tensor 1 ui u j ij ( ) 2 x j xi Elastic anisotropy birefringence ij cijkl kl Stress tensor 𝜎, Compliance C, Stiffness S Message: Wave propagation in anisotropic media is quite different from isotropic media: • There are in general 21 independent elastic constants (instead of 2 in the isotropic case), which can be reduced still further by considering the symmetry conditions found in different crystal structures. • There is shear wave splitting (analogous to optical birefringence, different polarizations in diff. directions) • Waves travel at different speeds depending in the direction of propagation abc 90o The cubic axes are equivalent, so the diagonal components for normal and shear distortions must be equal. And cubic is not elastically isotropic because a deformation along a cubic axis differs from the stress arising from a deformation along the diagonal. e.g., [100] vs. [111] Zener Anisotropy Ratio: A 2C44 C 44 C11 C12 x x=(a-b)/2 or C44 C11 C12 2