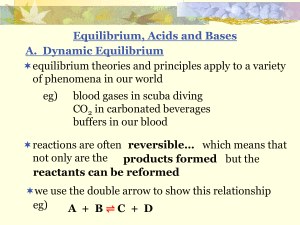
Section 4.8
... • Say you are asked how much CO2 is produced from the combustion of 15.0 moles of octane? • 2 C8H18 (l) + 25 O2 (g) → 16 CO2 (g) + 18 H2O (g) 16 mol CO 2 15.0 mol C 8H18 x 1.20 x 10 2 mol CO 2 2 mol C 8H18 ...
... • Say you are asked how much CO2 is produced from the combustion of 15.0 moles of octane? • 2 C8H18 (l) + 25 O2 (g) → 16 CO2 (g) + 18 H2O (g) 16 mol CO 2 15.0 mol C 8H18 x 1.20 x 10 2 mol CO 2 2 mol C 8H18 ...
A Low-Fluorine Solution with the F/Ba Mole Ratio of 2 for the
... deionized water, and propionic acid were mixed directly. The quantity of TFA was 10.3 mol.% of the total CH3 COO− anion with a uncertainty estimated to be 0.5%. After stirring for 1 h, the obtained solution was refined in a BUCHI Rotavapor R210 rotary evaporator under decompression for 2 h. Methanol ...
... deionized water, and propionic acid were mixed directly. The quantity of TFA was 10.3 mol.% of the total CH3 COO− anion with a uncertainty estimated to be 0.5%. After stirring for 1 h, the obtained solution was refined in a BUCHI Rotavapor R210 rotary evaporator under decompression for 2 h. Methanol ...
KHARKOV STATE MEDICAL UNIVERSITY
... Universities. The manual is written strictly in accordance with the prescribed syllabus. We feel convinced that the students face a lot of difficulty in understanding the language and the wide contents of the chemistry books. Therefore, we have made an attempt to bring out a simplified, helpful, com ...
... Universities. The manual is written strictly in accordance with the prescribed syllabus. We feel convinced that the students face a lot of difficulty in understanding the language and the wide contents of the chemistry books. Therefore, we have made an attempt to bring out a simplified, helpful, com ...
honors chemistry harvard-westlake second semester final exam
... a. How many moles of gas have been collected? b. Write the chemical reaction, including the correct numerical energy term on the appropriate side c. Assuming that the zinc has been completely consumed, how many grams of zinc were used in this reaction? d. If the resulting solution was evaporated, wh ...
... a. How many moles of gas have been collected? b. Write the chemical reaction, including the correct numerical energy term on the appropriate side c. Assuming that the zinc has been completely consumed, how many grams of zinc were used in this reaction? d. If the resulting solution was evaporated, wh ...
CHAPTER 4: CHEMICAL QUANTITIES and AQUEOUS REACTIONS
... 209 g of methanol (CH3OH) burns in air to give CO2 and H2O. What is the mass of water? 5. Write the equation CH3OH + O2 → CO2 + H2O 6. Balance the chemical equation 2CH3OH + 3O2 → 2CO2 + 4H2O 7. From the balanced equation, find out the moles of desired reactants and products. 2 moles of CH3OH gives ...
... 209 g of methanol (CH3OH) burns in air to give CO2 and H2O. What is the mass of water? 5. Write the equation CH3OH + O2 → CO2 + H2O 6. Balance the chemical equation 2CH3OH + 3O2 → 2CO2 + 4H2O 7. From the balanced equation, find out the moles of desired reactants and products. 2 moles of CH3OH gives ...
Modified ketone resin as an epoxy resin curing agent
... The C,H and Br contents of BCHF also agree with the predicted structure. The bromine content of BCHF polymer indicate that the bromination of terminal OH group could not be exist. This is only possible at higher temperature [11]. The reaction of BCHF with hydrazine and its various derivatives was ca ...
... The C,H and Br contents of BCHF also agree with the predicted structure. The bromine content of BCHF polymer indicate that the bromination of terminal OH group could not be exist. This is only possible at higher temperature [11]. The reaction of BCHF with hydrazine and its various derivatives was ca ...
TERMS AND DEFINITIONS IN THERMOCHEMISTRY
... This is the minimum amount of energy required to remove 1 mole of electrons from 1 mole of atoms or ions (as appropriate), both reactants and products being in the gaseous state. For example Na(g) nn> Na+(g) + e- ∆HI = 49$583 kJ mol-1 (18) 1st ionization energy of sodium Na+(g) nn> Na2+(g) + 2e- ∆HI ...
... This is the minimum amount of energy required to remove 1 mole of electrons from 1 mole of atoms or ions (as appropriate), both reactants and products being in the gaseous state. For example Na(g) nn> Na+(g) + e- ∆HI = 49$583 kJ mol-1 (18) 1st ionization energy of sodium Na+(g) nn> Na2+(g) + 2e- ∆HI ...
Unit 5 2 Thermodynamics Enthalpy
... When an element exists in more than one form under standard conditions (allotropy), the more stable form is used. Thus, O2(g) is assigned a ∆H°f = 0, whereas O3 and O are not. We can see this again and again in the literature, as graphite is considered to be the more thermodynamically stable isotope ...
... When an element exists in more than one form under standard conditions (allotropy), the more stable form is used. Thus, O2(g) is assigned a ∆H°f = 0, whereas O3 and O are not. We can see this again and again in the literature, as graphite is considered to be the more thermodynamically stable isotope ...
Answers - Pearson-Global
... d) In a sealed container, vapour particles in the space above the liquid return and stick to the surface of the liquid at the same rate as liquid particles are evaporating. ...
... d) In a sealed container, vapour particles in the space above the liquid return and stick to the surface of the liquid at the same rate as liquid particles are evaporating. ...
1970 - Warren County Schools
... 128.2). Calculate the molality of the pdichlorobenzene solution. 1984 C Give a scientific explanation for the following observa- (c) The freezing point of pure naphthalene is determined to be 80.2C. The solution prepared in (b) tions. Use equations or diagrams if they are relevant. is found to have ...
... 128.2). Calculate the molality of the pdichlorobenzene solution. 1984 C Give a scientific explanation for the following observa- (c) The freezing point of pure naphthalene is determined to be 80.2C. The solution prepared in (b) tions. Use equations or diagrams if they are relevant. is found to have ...
Quiz contsts questions chemistry
... 56 cm3 of oxygen combine with 112 cm3 of hydrogen to form water : When 56 cm3 of H2 is passed over heated capric oxide, the latter loses 0.04 g of its weight. All measurements are done under similar conditions of temperature and pressure (at. wt., H=1, O=16). Which of the following law is obeyed by ...
... 56 cm3 of oxygen combine with 112 cm3 of hydrogen to form water : When 56 cm3 of H2 is passed over heated capric oxide, the latter loses 0.04 g of its weight. All measurements are done under similar conditions of temperature and pressure (at. wt., H=1, O=16). Which of the following law is obeyed by ...
ENTHALPY CHANGE DH
... surroundings, therefore the enthalpy change, H, has a negative value. This is an exothermic reaction. If the system takes in heat energy during a reaction, enthalpy is gained from the surroundings, therefore the enthalpy change, H, has a positive value. This is an endothermic reaction. The size of ...
... surroundings, therefore the enthalpy change, H, has a negative value. This is an exothermic reaction. If the system takes in heat energy during a reaction, enthalpy is gained from the surroundings, therefore the enthalpy change, H, has a positive value. This is an endothermic reaction. The size of ...
1970 - 2005 Solids/Liquids/Solutions FRQs
... 128.2). Calculate the molality of the pdichlorobenzene solution. 1984 C Give a scientific explanation for the following observa- (c) The freezing point of pure naphthalene is determined to be 80.2C. The solution prepared in (b) tions. Use equations or diagrams if they are relevant. is found to have ...
... 128.2). Calculate the molality of the pdichlorobenzene solution. 1984 C Give a scientific explanation for the following observa- (c) The freezing point of pure naphthalene is determined to be 80.2C. The solution prepared in (b) tions. Use equations or diagrams if they are relevant. is found to have ...
Textbook sample chapter
... covalently bonded. Many of the formulae that you meet in this course have giant structures with ionic or covalent bonding. Sodium chloride has ionic bonding and consists of a large number of sodium ions and an equally large number of chloride ions held together in a lattice by electrostatic charges. ...
... covalently bonded. Many of the formulae that you meet in this course have giant structures with ionic or covalent bonding. Sodium chloride has ionic bonding and consists of a large number of sodium ions and an equally large number of chloride ions held together in a lattice by electrostatic charges. ...
The Major Classes of Chemical Reactions
... the amazing variety of chemical reactions. Rapid chemical changes occur among gas molecules as sunlight bathes the atmosphere or lightning rips through a stormy sky (see margin). Oceans are gigantic containers in which aqueous reaction chemistry goes on unceasingly. In every cell of your body, thous ...
... the amazing variety of chemical reactions. Rapid chemical changes occur among gas molecules as sunlight bathes the atmosphere or lightning rips through a stormy sky (see margin). Oceans are gigantic containers in which aqueous reaction chemistry goes on unceasingly. In every cell of your body, thous ...
Chapter 14
... Based on low temperature experiments, it appears that the entropy of every pure substance approaches the same value as T 0. K. Third law of thermodynamics: The absolute entropy (S) of a perfect crystal of any pure substance at absolute zero is 0.0 J/mol.K. Because there are standard ways of find ...
... Based on low temperature experiments, it appears that the entropy of every pure substance approaches the same value as T 0. K. Third law of thermodynamics: The absolute entropy (S) of a perfect crystal of any pure substance at absolute zero is 0.0 J/mol.K. Because there are standard ways of find ...
pdf version - Joliet Junior College
... Deviating from Raoult’s Law Solutions do not necessarily follow IDEAL (i.e. pure solute or solvent) behavior with regard to their Vapor Pressure v Composition plots – this is called deviating from Raoult’s Law. Discussion: What microscopic events would cause a deviation from Raoult’s Law, i.e. the ...
... Deviating from Raoult’s Law Solutions do not necessarily follow IDEAL (i.e. pure solute or solvent) behavior with regard to their Vapor Pressure v Composition plots – this is called deviating from Raoult’s Law. Discussion: What microscopic events would cause a deviation from Raoult’s Law, i.e. the ...
x - SharpSchool
... hydrogen. Evidence indicates that this reaction establishes an equilibrium with only partial conversion of reactants to products. Initially, 2.00 mol of each reactant is placed in the vessel. Kc for this reaction is 4.20 at 900C. Calculate the concentration of each substance at equilibrium. ...
... hydrogen. Evidence indicates that this reaction establishes an equilibrium with only partial conversion of reactants to products. Initially, 2.00 mol of each reactant is placed in the vessel. Kc for this reaction is 4.20 at 900C. Calculate the concentration of each substance at equilibrium. ...
CHAPTER 21 NONMETALLIC ELEMENTS AND THEIR COMPOUNDS
... molar masses. The molar mass of ammonium chloride is 53.5 g/mol, and the ratio of this to the molar mass of molecular hydrogen (2.02 g/mol) is 26.8. The experimental value of 14.5 is roughly half this amount. Such results usually indicate breakup or dissociation into smaller molecules in the gas pha ...
... molar masses. The molar mass of ammonium chloride is 53.5 g/mol, and the ratio of this to the molar mass of molecular hydrogen (2.02 g/mol) is 26.8. The experimental value of 14.5 is roughly half this amount. Such results usually indicate breakup or dissociation into smaller molecules in the gas pha ...
Sample Chapter - Chapter 4
... one atom attracts the electron pair more strongly than the other. For reasons discussed in Chapter 9, an O atom attracts electrons more strongly than an H atom. Therefore, in each of the O±H bonds of water, the electrons spend more time closer to the O (Figure 4.2B). This unequal distribution of the ...
... one atom attracts the electron pair more strongly than the other. For reasons discussed in Chapter 9, an O atom attracts electrons more strongly than an H atom. Therefore, in each of the O±H bonds of water, the electrons spend more time closer to the O (Figure 4.2B). This unequal distribution of the ...
A Few Things You Might Want To Know
... Mixtures can be heterogeneous or homogeneous (= solutions). They consist of substances that can be separated by physical changes (distillation, crystallization, chromatography). Substances can be either elements or compounds. Compounds can be separated into elements by chemical changes (redox reacti ...
... Mixtures can be heterogeneous or homogeneous (= solutions). They consist of substances that can be separated by physical changes (distillation, crystallization, chromatography). Substances can be either elements or compounds. Compounds can be separated into elements by chemical changes (redox reacti ...
Stoichiometry notes 1
... 2. Label your given and target substances. 3. Convert your given unit(s) to moles of given substance using the appropriate conversion factor. 4. Convert moles of given substance to moles of target substance using the mole ratio from the balanced equation. 5. Convert moles of target substance to the ...
... 2. Label your given and target substances. 3. Convert your given unit(s) to moles of given substance using the appropriate conversion factor. 4. Convert moles of given substance to moles of target substance using the mole ratio from the balanced equation. 5. Convert moles of target substance to the ...
ХИМИЯ НА АНГЛИЙСКОМ ЯЗЫКЕ
... Determine whether there is a significant difference between the mean and the expected value at α = 0.05. 1.42. A 2.6540-g sample of an iron ore known to contain 53.51% m/m Fe is dissolved in a small portion of concentrated HCl and diluted to volume in a 250-mL volumetric flask. A spectrophotometric ...
... Determine whether there is a significant difference between the mean and the expected value at α = 0.05. 1.42. A 2.6540-g sample of an iron ore known to contain 53.51% m/m Fe is dissolved in a small portion of concentrated HCl and diluted to volume in a 250-mL volumetric flask. A spectrophotometric ...
The Major Classes of Chemical Reactions
... Chemists use three types of equations to represent aqueous ionic reactions: molecular, total ionic, and net ionic equations. As you’ll see in the two types of ionic equations, by balancing the atoms, we also balance the charges. Let’s examine a reaction to see what each of these equations shows. Whe ...
... Chemists use three types of equations to represent aqueous ionic reactions: molecular, total ionic, and net ionic equations. As you’ll see in the two types of ionic equations, by balancing the atoms, we also balance the charges. Let’s examine a reaction to see what each of these equations shows. Whe ...
Thermometric titration

A thermometric titration is one of a number of instrumental titration techniques where endpoints can be located accurately and precisely without a subjective interpretation on the part of the analyst as to their location. Enthalpy change is arguably the most fundamental and universal property of chemical reactions, so the observation of temperature change is a natural choice in monitoring their progress. It is not a new technique, with possibly the first recognizable thermometric titration method reported early in the 20th century (Bell and Cowell, 1913). In spite of its attractive features, and in spite of the considerable research that has been conducted in the field and a large body of applications that have been developed; it has been until now an under-utilized technique in the critical area of industrial process and quality control. Automated potentiometric titration systems have pre-dominated in this area since the 1970s. With the advent of cheap computers able to handle the powerful thermometric titration software, development has now reached the stage where easy to use automated thermometric titration systems can in many cases offer a superior alternative to potentiometric titrimetry.The applications of thermometric titrimetry discussed on this page are by no means exhaustive. The reader is referred to the bibliography for further reading on the subject.