To do List
... example would be NaHCO3. What is the term that is used to describe a metal's ability to be drawn into a wire? The Tyndall effect is a characteristic of what classification of matter? ...
... example would be NaHCO3. What is the term that is used to describe a metal's ability to be drawn into a wire? The Tyndall effect is a characteristic of what classification of matter? ...
Stoichiometry - coercingmolecules
... by counting or weighing them, depending on which method is more convenient ...
... by counting or weighing them, depending on which method is more convenient ...
Chapter 8
... In most chemical reactions several reactants combine to form products. As soon as one of the reactants runs out, the reaction will stop, even if the other reactants are still present. We define the limiting reactant as the reactant the first runs out in a chemical reaction. Note that the theoretical ...
... In most chemical reactions several reactants combine to form products. As soon as one of the reactants runs out, the reaction will stop, even if the other reactants are still present. We define the limiting reactant as the reactant the first runs out in a chemical reaction. Note that the theoretical ...
1.8 M - Thierry Karsenti
... and export them as consistently styled content within Learning Objects. This frees the content developer to focus on the quality of the content without having to overly concern themselves with presentation. Similarly, editors of learning objects need not concern themselves with ensuring authors use ...
... and export them as consistently styled content within Learning Objects. This frees the content developer to focus on the quality of the content without having to overly concern themselves with presentation. Similarly, editors of learning objects need not concern themselves with ensuring authors use ...
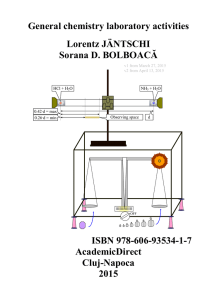
General chemistry laboratory activities, Lorentz
... two both in a reaction under vacuum, and pressure, sometimes simultaneously. Varieties include multiple neck flasks (can have two to five, or less commonly, six necks, each topped by ground glass connections) being used in more complex reactions that require the controlled mixing of multiple reagent ...
... two both in a reaction under vacuum, and pressure, sometimes simultaneously. Varieties include multiple neck flasks (can have two to five, or less commonly, six necks, each topped by ground glass connections) being used in more complex reactions that require the controlled mixing of multiple reagent ...
2001 AP Chemistry Scoring Guidelines - AP Central
... The College Board is a national nonprofit membership association dedicated to preparing, inspiring, and connecting students to college and opportunity. Founded in 1900, the association is composed of more than 3,900 schools, colleges, universities, and other educational organizations. Each year, the ...
... The College Board is a national nonprofit membership association dedicated to preparing, inspiring, and connecting students to college and opportunity. Founded in 1900, the association is composed of more than 3,900 schools, colleges, universities, and other educational organizations. Each year, the ...
Chapter 4
... 4.1 General Properties of Aqueous Solutions A solution is a homogeneous mixture of two or more substances. The solute is the substance present in a smaller amount, and the solvent is the substance present in a larger amount. A solution may be gaseous (such as air), solid (such as an alloy), or liqui ...
... 4.1 General Properties of Aqueous Solutions A solution is a homogeneous mixture of two or more substances. The solute is the substance present in a smaller amount, and the solvent is the substance present in a larger amount. A solution may be gaseous (such as air), solid (such as an alloy), or liqui ...
Vinnitsa National Pirogov Memorial Medical University Biological
... 1.Write the electronic structure of potassium atom and ion. 2. Write the electronic structure of aluminium atom and Al3+ ion. 3.Write the equations of the below given chain. ...
... 1.Write the electronic structure of potassium atom and ion. 2. Write the electronic structure of aluminium atom and Al3+ ion. 3.Write the equations of the below given chain. ...
WJEC Eduqas A Level Chemistry specification
... included in the overview will not be directly assessed. Practical work is an intrinsic part of this specification. It is vitally important in developing a conceptual understanding of many topics and it enhances the experience and enjoyment of chemistry. The practical skills developed are also fundam ...
... included in the overview will not be directly assessed. Practical work is an intrinsic part of this specification. It is vitally important in developing a conceptual understanding of many topics and it enhances the experience and enjoyment of chemistry. The practical skills developed are also fundam ...
GCE Chemistry Specification (From 2015 - WALES ONLY
... Medicine, Biochemistry and Chemical Engineering. It also develops a range of knowledge and skills essential for direct entry into employment in many chemistryrelated fields. In addition, the specification provides a coherent, satisfying and worthwhile course of study for candidates who do not progre ...
... Medicine, Biochemistry and Chemical Engineering. It also develops a range of knowledge and skills essential for direct entry into employment in many chemistryrelated fields. In addition, the specification provides a coherent, satisfying and worthwhile course of study for candidates who do not progre ...
F:\Users\Steven\Documents\Chemistry\CHEM120\Problem Set
... When I went to write this problem I looked at the periodic table and saw that Rubidium had a mass of 85.467. Since the mass of all isotopes are even (or nearly so) and this average was uneven I knew immediately that rubidium had to have two major isotopes. When I looked up the isotopes sure enough t ...
... When I went to write this problem I looked at the periodic table and saw that Rubidium had a mass of 85.467. Since the mass of all isotopes are even (or nearly so) and this average was uneven I knew immediately that rubidium had to have two major isotopes. When I looked up the isotopes sure enough t ...
unit iv – stoichiometry 1
... XI. Concentration and Dilution of Solutions A. Concentration of Solutions – most often, we express it as molarity (moles solute / liters sol’n) 1) What is the molarity of a solution in which you dissolve 10.0g of glucose (C6H12O6) in 50θ mL of water? ...
... XI. Concentration and Dilution of Solutions A. Concentration of Solutions – most often, we express it as molarity (moles solute / liters sol’n) 1) What is the molarity of a solution in which you dissolve 10.0g of glucose (C6H12O6) in 50θ mL of water? ...
sample chapter
... Many chemical reactions and virtually all biological processes take place in an aqueous environment. Therefore, it is important to understand the properties of different substances in solution with water. To start with, what exactly is a solution? A solution is a homogeneous mixture of two or more s ...
... Many chemical reactions and virtually all biological processes take place in an aqueous environment. Therefore, it is important to understand the properties of different substances in solution with water. To start with, what exactly is a solution? A solution is a homogeneous mixture of two or more s ...
ExamView - 1999 AP Chemistry Exam.tst
... A) equilibrium water vapor pressure is higher due to the higher atmospheric pressure B) equilibrium water vapor pressure is lower due to the higher atmospheric pressure C) equilibrium water vapor pressure equals the atmospheric pressure at a lower temperature D) water molecules have a higher average ...
... A) equilibrium water vapor pressure is higher due to the higher atmospheric pressure B) equilibrium water vapor pressure is lower due to the higher atmospheric pressure C) equilibrium water vapor pressure equals the atmospheric pressure at a lower temperature D) water molecules have a higher average ...
tro2_ppt_lecture_04 - Louisiana Tech University
... Problem Strategy: Determine how many grams of glucose (C6H6O6) would be produced by the plant if 37.8 grams of CO2 is consumed. 1. Need a balanced equation: 6 CO2(g) + 6 H2O(l) C6H6O6(s) + 6 O2(g) 2. Determine moles of CO2 consumed. 37.8 g CO2 x (1 mol CO2/44.0 g) = 0.859 mol CO2 3. Determine how ...
... Problem Strategy: Determine how many grams of glucose (C6H6O6) would be produced by the plant if 37.8 grams of CO2 is consumed. 1. Need a balanced equation: 6 CO2(g) + 6 H2O(l) C6H6O6(s) + 6 O2(g) 2. Determine moles of CO2 consumed. 37.8 g CO2 x (1 mol CO2/44.0 g) = 0.859 mol CO2 3. Determine how ...
Stoichiometry1
... The lower amount of a product is the correct answer. The reactant that makes the least amount of product is the limiting reactant. Once you determine the limiting reactant, you should ALWAYS start with it! Be sure to pick a product! You can’t compare to see which is greater and which is lower ...
... The lower amount of a product is the correct answer. The reactant that makes the least amount of product is the limiting reactant. Once you determine the limiting reactant, you should ALWAYS start with it! Be sure to pick a product! You can’t compare to see which is greater and which is lower ...
Chemistry 1250 - Sp17 Solutions for Midterm 1
... This is a limiting reactant problem. These are just stoichiometry problems. There is more than one way to do a LR problem. In this case it’s asking for the limiting reactant and the mass of excess reactant remaining after completion of the reaction. 2 Fe(OH)3 (s) + 3 H2SO4 (aq) v Fe2(SO4)3 (aq) + 6 ...
... This is a limiting reactant problem. These are just stoichiometry problems. There is more than one way to do a LR problem. In this case it’s asking for the limiting reactant and the mass of excess reactant remaining after completion of the reaction. 2 Fe(OH)3 (s) + 3 H2SO4 (aq) v Fe2(SO4)3 (aq) + 6 ...
Principles of Chemistry 1 and 2 Notes
... 2) In molecules with more than two atoms (three atoms and more), the polarity of bonds and the molecular geometry determine whether or not the molecule has a dipole moment or not. Example 1: CO2 ::O = C = O:: (linear, according to the VSEPR model) The CO2 compound does have two electronegative atoms ...
... 2) In molecules with more than two atoms (three atoms and more), the polarity of bonds and the molecular geometry determine whether or not the molecule has a dipole moment or not. Example 1: CO2 ::O = C = O:: (linear, according to the VSEPR model) The CO2 compound does have two electronegative atoms ...
5. Homework 5-Answers
... D) Energy is being created as time passes. We have more energy in the universe now than when time began. Ans: A 29. A gas is compressed in a cylinder from a volume of 20.0 L to 2.0 L by a constant pressure of 10.0 atm. Calculate the amount of work done on the system. A) 1.01 104 J B) –180 J C) 1.8 ...
... D) Energy is being created as time passes. We have more energy in the universe now than when time began. Ans: A 29. A gas is compressed in a cylinder from a volume of 20.0 L to 2.0 L by a constant pressure of 10.0 atm. Calculate the amount of work done on the system. A) 1.01 104 J B) –180 J C) 1.8 ...
Document
... #1. In terms of Particles An Element is made of atoms A covalent compound (made of only nonmetals) is made up of molecules (Don’t forget the diatomic elements) Ionic Compounds (made of a metal and nonmetal parts) are made of formula units ...
... #1. In terms of Particles An Element is made of atoms A covalent compound (made of only nonmetals) is made up of molecules (Don’t forget the diatomic elements) Ionic Compounds (made of a metal and nonmetal parts) are made of formula units ...
CB document - mvhs
... which units they should be using. I would emphasize with teachers to always have students include units in their work, even though there are times the units are ignored when reading the test. For example, if the test says to solve in kilojoules, any number written is assumed to be in kJ if no unit i ...
... which units they should be using. I would emphasize with teachers to always have students include units in their work, even though there are times the units are ignored when reading the test. For example, if the test says to solve in kilojoules, any number written is assumed to be in kJ if no unit i ...
Acidic Environment
... Pressure is increased is by reducing the volume, or decrease by increasing the volume. ...
... Pressure is increased is by reducing the volume, or decrease by increasing the volume. ...
STOICHIOMETRY via ChemLog - Small
... When carrying out a chemical reaction, we may use the exact amount of each reactant needed. Or, we may use an excess of some reactants and a limited amount of others. We may do this if one reactant is very expensive and others are inexpensive so that we can use all of the expensive compound. It can ...
... When carrying out a chemical reaction, we may use the exact amount of each reactant needed. Or, we may use an excess of some reactants and a limited amount of others. We may do this if one reactant is very expensive and others are inexpensive so that we can use all of the expensive compound. It can ...
Chapter 1 – Reaction Kinetics Answer Key
... 3. The concentrations of pure solids and liquids are fixed. That is they do not change (appreciably for the liquid if it is the solvent and at all for the solid) during a chemical reaction. ...
... 3. The concentrations of pure solids and liquids are fixed. That is they do not change (appreciably for the liquid if it is the solvent and at all for the solid) during a chemical reaction. ...
Thermometric titration

A thermometric titration is one of a number of instrumental titration techniques where endpoints can be located accurately and precisely without a subjective interpretation on the part of the analyst as to their location. Enthalpy change is arguably the most fundamental and universal property of chemical reactions, so the observation of temperature change is a natural choice in monitoring their progress. It is not a new technique, with possibly the first recognizable thermometric titration method reported early in the 20th century (Bell and Cowell, 1913). In spite of its attractive features, and in spite of the considerable research that has been conducted in the field and a large body of applications that have been developed; it has been until now an under-utilized technique in the critical area of industrial process and quality control. Automated potentiometric titration systems have pre-dominated in this area since the 1970s. With the advent of cheap computers able to handle the powerful thermometric titration software, development has now reached the stage where easy to use automated thermometric titration systems can in many cases offer a superior alternative to potentiometric titrimetry.The applications of thermometric titrimetry discussed on this page are by no means exhaustive. The reader is referred to the bibliography for further reading on the subject.