Sample Paper - Army Public School Jammu Cantt
... Use of calculators is not allowed, use log tables wherever required. 1. Name the non stoichiometric point defect responsible for colour in alkali metal halides. 2. What is shape selective catalysis? 3. Amongst the isomeric alkanes of molecular formula C5H12, identify the one that on photochemical ch ...
... Use of calculators is not allowed, use log tables wherever required. 1. Name the non stoichiometric point defect responsible for colour in alkali metal halides. 2. What is shape selective catalysis? 3. Amongst the isomeric alkanes of molecular formula C5H12, identify the one that on photochemical ch ...
Exam practice answers
... Le Chatelier’s principle states that if a closed system under equilibrium is subject to a change, the system will move in such a way as to minimise the effect of the change. (iii) Temperature: the forward reaction is exothermic by low temperature . pressure ...
... Le Chatelier’s principle states that if a closed system under equilibrium is subject to a change, the system will move in such a way as to minimise the effect of the change. (iii) Temperature: the forward reaction is exothermic by low temperature . pressure ...
CHEMISTRY: Practice Spring Final
... 16) Calculate the new pressure of helium gas in a balloon if the original volume of 2.5 L at 100.0 kPa increases to 18 L. A) 0.45 kPa B) 720 kPa C) 14 kPa D) 13 kPa 17) Which of the following is not a basic assumption of kinetic theory? A) Gases are composed of particles. B) The particles of all mov ...
... 16) Calculate the new pressure of helium gas in a balloon if the original volume of 2.5 L at 100.0 kPa increases to 18 L. A) 0.45 kPa B) 720 kPa C) 14 kPa D) 13 kPa 17) Which of the following is not a basic assumption of kinetic theory? A) Gases are composed of particles. B) The particles of all mov ...
Test 2
... Show your work for complete (and partial) credit. Report your answers to the correct number of significant figures, and use units where appropriate. All chemical equations should balance; indicate phases. NA=6.022x1023 1. What is the definition of the mole? ...
... Show your work for complete (and partial) credit. Report your answers to the correct number of significant figures, and use units where appropriate. All chemical equations should balance; indicate phases. NA=6.022x1023 1. What is the definition of the mole? ...
Second review [Compatibility Mode]
... When 0.0300 mol of Na was added to 100.0 g of water, the temperature of the resulting solution rose from 25.0 oC to 37.9 oC. If the specific heat of the solution was 4.18 J g-1 K-1, calculate ? H, in kJ, for the reaction as written. ...
... When 0.0300 mol of Na was added to 100.0 g of water, the temperature of the resulting solution rose from 25.0 oC to 37.9 oC. If the specific heat of the solution was 4.18 J g-1 K-1, calculate ? H, in kJ, for the reaction as written. ...
PDF (Size: 41K)
... Explain, with reference to the standard electrode potential for sodium and hydrogen, why sodium is manufactured using this method rather than by the electrolysis of aqueous sodium chloride. Na+(aq) + e– ...
... Explain, with reference to the standard electrode potential for sodium and hydrogen, why sodium is manufactured using this method rather than by the electrolysis of aqueous sodium chloride. Na+(aq) + e– ...
Solution
... 19) Novocaine can be used as a local anesthetic, and has a pKb of 5.00. What is the ratio of novocaine to its conjugate acid if a small amount is added to the blood, which has a pH of approximately 7? A) 7/5 B) 5/7 C) 1/1 D) 1/100 E) 100/1 For questions 20-23, choose from the following graphs to an ...
... 19) Novocaine can be used as a local anesthetic, and has a pKb of 5.00. What is the ratio of novocaine to its conjugate acid if a small amount is added to the blood, which has a pH of approximately 7? A) 7/5 B) 5/7 C) 1/1 D) 1/100 E) 100/1 For questions 20-23, choose from the following graphs to an ...
Unit 5 Practice Problems (with answers at end) - H
... room temperature? 15. Calculate So for the reaction above. What does this value suggest? Why is the sign what it is? 16. Assuming the temperature is 25oC, calculate Ho for the reaction above, using the results in the previous problems. Since the entropy decreases for the system, how can you accoun ...
... room temperature? 15. Calculate So for the reaction above. What does this value suggest? Why is the sign what it is? 16. Assuming the temperature is 25oC, calculate Ho for the reaction above, using the results in the previous problems. Since the entropy decreases for the system, how can you accoun ...
Title - Iowa State University
... 16) How many moles of sulfur are present in 45g of Al2(SO4)3? (MM: 342g/mol) a)0.132 moles of S b) 0.392 moles of S c)0.78moles of S ...
... 16) How many moles of sulfur are present in 45g of Al2(SO4)3? (MM: 342g/mol) a)0.132 moles of S b) 0.392 moles of S c)0.78moles of S ...
study guide and review for first semester final
... Ex. Cu + HNO3 Cu(NO3)2 + NO + H2O 21. Be able to balance redox equations for basic or acidic reactions using the ion electron method. Ex. Cr2O7-2 + Fe+2 Cr+3 + Fe+3 (acid solution) Ex. SO3-2 + MnO4-1 SO4-2 + MnO2 ...
... Ex. Cu + HNO3 Cu(NO3)2 + NO + H2O 21. Be able to balance redox equations for basic or acidic reactions using the ion electron method. Ex. Cr2O7-2 + Fe+2 Cr+3 + Fe+3 (acid solution) Ex. SO3-2 + MnO4-1 SO4-2 + MnO2 ...
10 TEST 2 (of 3)
... Complete the following using the words ENDOTHERMIC, ENTHALPY, EXOTHERMIC (a) The heat evolved in a chemical reaction at constant pressure is called the change in __________________ for the reaction (ΔH). In an ______________ reaction ΔH is positive, in an _______________ reaction ΔH Is negative. ...
... Complete the following using the words ENDOTHERMIC, ENTHALPY, EXOTHERMIC (a) The heat evolved in a chemical reaction at constant pressure is called the change in __________________ for the reaction (ΔH). In an ______________ reaction ΔH is positive, in an _______________ reaction ΔH Is negative. ...
Advanced Placement Chemistry: 1984 Free Response Questions
... 2) For a hypothetical chemical reaction that has the stoichiometry 2 X + Y ---> Z, the following initial rate data were obtained. All the measurements were made at the same temperature. Initial Rate of Formation of Z, ...
... 2) For a hypothetical chemical reaction that has the stoichiometry 2 X + Y ---> Z, the following initial rate data were obtained. All the measurements were made at the same temperature. Initial Rate of Formation of Z, ...
PRACTICE * Naming and Writing Ionic Compounds
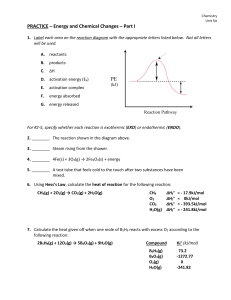
... 1. Label each area on the reaction diagram with the appropriate letters listed below. Not all letters will be used. A. ...
... 1. Label each area on the reaction diagram with the appropriate letters listed below. Not all letters will be used. A. ...
Thermometric titration

A thermometric titration is one of a number of instrumental titration techniques where endpoints can be located accurately and precisely without a subjective interpretation on the part of the analyst as to their location. Enthalpy change is arguably the most fundamental and universal property of chemical reactions, so the observation of temperature change is a natural choice in monitoring their progress. It is not a new technique, with possibly the first recognizable thermometric titration method reported early in the 20th century (Bell and Cowell, 1913). In spite of its attractive features, and in spite of the considerable research that has been conducted in the field and a large body of applications that have been developed; it has been until now an under-utilized technique in the critical area of industrial process and quality control. Automated potentiometric titration systems have pre-dominated in this area since the 1970s. With the advent of cheap computers able to handle the powerful thermometric titration software, development has now reached the stage where easy to use automated thermometric titration systems can in many cases offer a superior alternative to potentiometric titrimetry.The applications of thermometric titrimetry discussed on this page are by no means exhaustive. The reader is referred to the bibliography for further reading on the subject.



![Second review [Compatibility Mode]](http://s1.studyres.com/store/data/003692853_1-a578e4717b0c8365c11d7e7f576654ae-300x300.png)