Worked solutions to the problems
... have included the list of topics that are generally accepted as the basis for examination questions for the International Chemistry Olympiad - this is the same list of topics that we inherited from Montreal. More importantly, the problems have been designed to challenge and stimulate the interests o ...
... have included the list of topics that are generally accepted as the basis for examination questions for the International Chemistry Olympiad - this is the same list of topics that we inherited from Montreal. More importantly, the problems have been designed to challenge and stimulate the interests o ...
Chapter 09 An Overview of Chemical Reactions Notes
... Precipitation Reaction: - a reaction where a precipitate (new solid) is formed as a product. Neutralization Reaction: - a reaction between an acid and a base where water is formed as a product. To Predict Products and Balance Chemical Equations: 1. Write the correct chemical formulas for all product ...
... Precipitation Reaction: - a reaction where a precipitate (new solid) is formed as a product. Neutralization Reaction: - a reaction between an acid and a base where water is formed as a product. To Predict Products and Balance Chemical Equations: 1. Write the correct chemical formulas for all product ...
Chapter 10
... possible based on amount of reactant(s). Actual Yield = How much product was actually obtained. ...
... possible based on amount of reactant(s). Actual Yield = How much product was actually obtained. ...
4Chemical Quantities and Aqueous Reactions
... like the glass of a greenhouse, allowing sunlight to enter the atmosphere and warm Earth’s surface, but preventing some of the heat generated by the sunlight from escaping, as shown in Figure 4.1▲. The balance between incoming and outgoing energy from the sun determines Earth’s average temperature. ...
... like the glass of a greenhouse, allowing sunlight to enter the atmosphere and warm Earth’s surface, but preventing some of the heat generated by the sunlight from escaping, as shown in Figure 4.1▲. The balance between incoming and outgoing energy from the sun determines Earth’s average temperature. ...
Chapter 19: Acids and Bases
... hydrangea can vary from pink to blue depending upon the acidity of the soil in which it is grown. ...
... hydrangea can vary from pink to blue depending upon the acidity of the soil in which it is grown. ...
Chemistry 134 Problem Set Introduction
... 14.61 (a) Write the electron configuration for a silicon atom. (b) Draw an orbital diagram for a silicon atom. (c) Assign a possible set of four quantum numbers for each of the electrons in the valence shell of a silicon atom. 14.62 Ethane (C2H6) is a flammable gas. It will burn in oxygen gas to pr ...
... 14.61 (a) Write the electron configuration for a silicon atom. (b) Draw an orbital diagram for a silicon atom. (c) Assign a possible set of four quantum numbers for each of the electrons in the valence shell of a silicon atom. 14.62 Ethane (C2H6) is a flammable gas. It will burn in oxygen gas to pr ...
Tro Chemistry a Molecular Approach, 3E
... The generation of the electricity used by a medium-sized home produces about 16 kg of NO2 per year. Assuming that there is adequate O2 and H2O, what mass of HNO3, in kg, can form from this amount of NO2 pollutant? ...
... The generation of the electricity used by a medium-sized home produces about 16 kg of NO2 per year. Assuming that there is adequate O2 and H2O, what mass of HNO3, in kg, can form from this amount of NO2 pollutant? ...
Student Study Guide Chemistry 534
... by an ocean of gas called the atmosphere, many of the properties of gases are already familiar to us. We know that we can squeeze a balloon into a smaller shape and that perfume released into the corner of a room can, in time, be detected all over the room even if the air is still. Gases such as car ...
... by an ocean of gas called the atmosphere, many of the properties of gases are already familiar to us. We know that we can squeeze a balloon into a smaller shape and that perfume released into the corner of a room can, in time, be detected all over the room even if the air is still. Gases such as car ...
1.4 Enthalpy
... Most reactions do not occur 'spontaneously' but need a little bit of energy to get them going, a spark is needed to set methane alight. This 'bit of energy' is called the activation energy. It is the energy required to break the bonds in the reactants. Exothermic reactions: ...
... Most reactions do not occur 'spontaneously' but need a little bit of energy to get them going, a spark is needed to set methane alight. This 'bit of energy' is called the activation energy. It is the energy required to break the bonds in the reactants. Exothermic reactions: ...
Ch. 12 Stoichiometry
... terms of different quantities such as number of atoms, molecules or moles; mass; and volume. ...
... terms of different quantities such as number of atoms, molecules or moles; mass; and volume. ...
1. Atomic Structure and Periodic Table THE MASS SPECTROMETER
... therefore attracted much more strongly by the nucleus than the fourth electron. It also does not have any shielding by inner complete shells of electron ...
... therefore attracted much more strongly by the nucleus than the fourth electron. It also does not have any shielding by inner complete shells of electron ...
Chemical Quantities(mole).
... Ideal gas Law: PV = nRT P = pressure (kPa) V = volume (Liters) n = 1 mole R = constant value (8.31 kPa ∙ L) mol ∙ K T = temperature (Kelvin) ...
... Ideal gas Law: PV = nRT P = pressure (kPa) V = volume (Liters) n = 1 mole R = constant value (8.31 kPa ∙ L) mol ∙ K T = temperature (Kelvin) ...
Chemical Quantities and Aqueous Reactions
... The balanced chemical equation shows that 16 CO2 molecules are produced for every 2 molecules of octane burned. This numerical relationship between molecules can be extended to the amounts in moles as follows: The coefficients in a chemical reaction specify the relative amounts in moles of each of t ...
... The balanced chemical equation shows that 16 CO2 molecules are produced for every 2 molecules of octane burned. This numerical relationship between molecules can be extended to the amounts in moles as follows: The coefficients in a chemical reaction specify the relative amounts in moles of each of t ...
Equilibrium Booklet - mrstorie
... 1. The formation of ammonia from hydrogen and nitrogen occurs by the reaction below: 3 H2 (g) + N2 (g) ↔ 2 NH3 (g) Analysis of an equilibrium mixture of nitrogen, hydrogen, and ammonia contained in a 1.00 L flask at 300.0°C gives the following results: hydrogen 0.150 moles; nitrogen 0.250 moles: amm ...
... 1. The formation of ammonia from hydrogen and nitrogen occurs by the reaction below: 3 H2 (g) + N2 (g) ↔ 2 NH3 (g) Analysis of an equilibrium mixture of nitrogen, hydrogen, and ammonia contained in a 1.00 L flask at 300.0°C gives the following results: hydrogen 0.150 moles; nitrogen 0.250 moles: amm ...
9/11/01
... But what if we had only gotten 100. g of SO 2 with those amounts of CS2 and O2? - reaction did not go to completion - several competing reactions involving CS2 and/or O2 were going on at the same time. Theoretical yield – the amount of product we should get when a reactant is completely consumed acc ...
... But what if we had only gotten 100. g of SO 2 with those amounts of CS2 and O2? - reaction did not go to completion - several competing reactions involving CS2 and/or O2 were going on at the same time. Theoretical yield – the amount of product we should get when a reactant is completely consumed acc ...
4U Chemistry Practice Exam - Coristines
... 73. In an experiment, strips of three metalsX, Y, and Zwere placed in beakers that contained solutions of the aqueous ions X2, Y2, and Z2and were allowed to react. The following data were obtained. Based on these data, what is the order of the metal ions, in decreasing ability to be reduced. ...
... 73. In an experiment, strips of three metalsX, Y, and Zwere placed in beakers that contained solutions of the aqueous ions X2, Y2, and Z2and were allowed to react. The following data were obtained. Based on these data, what is the order of the metal ions, in decreasing ability to be reduced. ...
Acids, Bases and Salts
... of positively charged cations (usually metal or ammonium ions) and the negatively charged anions, so that the product remains neutral and without a net charge. The anions may be inorganic (Cl-) as well as organic (CH3COO-) and monoatomic (F-) as well as polyatomic ions (SO42-). Salt's solution in wa ...
... of positively charged cations (usually metal or ammonium ions) and the negatively charged anions, so that the product remains neutral and without a net charge. The anions may be inorganic (Cl-) as well as organic (CH3COO-) and monoatomic (F-) as well as polyatomic ions (SO42-). Salt's solution in wa ...
AP Chemistry Notes and Worksheets 2014
... o Unfortunately, you can't deduce absolute formulas from this data. o Shows that each element consists of a certain type of atom and that compounds were formed from specific combinations of atoms. 2.3 Dalton’s Atomic Theory Dalton's Atomic Theory -1808 o Each element is made up of particles called ...
... o Unfortunately, you can't deduce absolute formulas from this data. o Shows that each element consists of a certain type of atom and that compounds were formed from specific combinations of atoms. 2.3 Dalton’s Atomic Theory Dalton's Atomic Theory -1808 o Each element is made up of particles called ...
Chapter 3 Stoichiometry STOICHIOMETRY: The chemical arithmetic
... the way we expect them to • Using stoichiometry we can calculate the theoretical (Maximum) amount of product formed in a reaction. ...
... the way we expect them to • Using stoichiometry we can calculate the theoretical (Maximum) amount of product formed in a reaction. ...
Chapter 4: Types of Chemical Reactions and Solution Stoichiometry
... Example: If a solution containing potassium chloride is added to a solution containing ammonium nitrate, will a precipitate form? KCl(aq) + NH4NO3(aq) → K+(aq) + Cl-(aq) + NH4+(aq) + NO3-(aq) Possible reaction products are KCl and NH4NO3, NH4Cl and KNO3. All are soluble, so there is no precipitate. ...
... Example: If a solution containing potassium chloride is added to a solution containing ammonium nitrate, will a precipitate form? KCl(aq) + NH4NO3(aq) → K+(aq) + Cl-(aq) + NH4+(aq) + NO3-(aq) Possible reaction products are KCl and NH4NO3, NH4Cl and KNO3. All are soluble, so there is no precipitate. ...
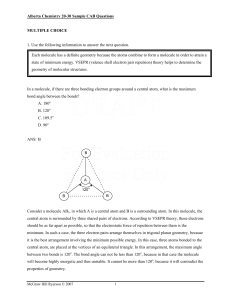
Alberta Chemistry 20-30 Sample CAB Questions - McGraw
... Consider a molecule AB3, in which A is a central atom and B is a surrounding atom. In this molecule, the central atom is surrounded by three shared pairs of electrons. According to VSEPR theory, these electrons should be as far apart as possible, so that the electrostatic force of repulsion between ...
... Consider a molecule AB3, in which A is a central atom and B is a surrounding atom. In this molecule, the central atom is surrounded by three shared pairs of electrons. According to VSEPR theory, these electrons should be as far apart as possible, so that the electrostatic force of repulsion between ...
STOICHIOMETRY
... theoretical yield Often, either accidentally or deliberately, one of the reagents in a reaction is present in excess while another reagent is the limiting reagent, i.e., there is not enough of it to use up all the reagent which is in excess. Only the quantity of limiting reagent can be used to d ...
... theoretical yield Often, either accidentally or deliberately, one of the reagents in a reaction is present in excess while another reagent is the limiting reagent, i.e., there is not enough of it to use up all the reagent which is in excess. Only the quantity of limiting reagent can be used to d ...
electrical energy and capacitance
... Chemical equations are quantitative because they tell us how many reactants and products interact in a given reaction. In particular, chemical reactions are written in mole to mole ratios. For example, 3 H2(g) + N2(g) 2 NH3(g) means that 3 moles of hydrogen gas react with 1 mole of nitrogen gas to ...
... Chemical equations are quantitative because they tell us how many reactants and products interact in a given reaction. In particular, chemical reactions are written in mole to mole ratios. For example, 3 H2(g) + N2(g) 2 NH3(g) means that 3 moles of hydrogen gas react with 1 mole of nitrogen gas to ...
To do List
... example would be NaHCO3. What is the term that is used to describe a metal's ability to be drawn into a wire? The Tyndall effect is a characteristic of what classification of matter? ...
... example would be NaHCO3. What is the term that is used to describe a metal's ability to be drawn into a wire? The Tyndall effect is a characteristic of what classification of matter? ...
Thermometric titration

A thermometric titration is one of a number of instrumental titration techniques where endpoints can be located accurately and precisely without a subjective interpretation on the part of the analyst as to their location. Enthalpy change is arguably the most fundamental and universal property of chemical reactions, so the observation of temperature change is a natural choice in monitoring their progress. It is not a new technique, with possibly the first recognizable thermometric titration method reported early in the 20th century (Bell and Cowell, 1913). In spite of its attractive features, and in spite of the considerable research that has been conducted in the field and a large body of applications that have been developed; it has been until now an under-utilized technique in the critical area of industrial process and quality control. Automated potentiometric titration systems have pre-dominated in this area since the 1970s. With the advent of cheap computers able to handle the powerful thermometric titration software, development has now reached the stage where easy to use automated thermometric titration systems can in many cases offer a superior alternative to potentiometric titrimetry.The applications of thermometric titrimetry discussed on this page are by no means exhaustive. The reader is referred to the bibliography for further reading on the subject.