* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Stoichiometry notes 1
Debye–Hückel equation wikipedia , lookup
Electrolysis of water wikipedia , lookup
Computational chemistry wikipedia , lookup
Stöber process wikipedia , lookup
Kinetic resolution wikipedia , lookup
Liquid–liquid extraction wikipedia , lookup
Relativistic quantum mechanics wikipedia , lookup
Gas chromatography–mass spectrometry wikipedia , lookup
Chemical equilibrium wikipedia , lookup
Size-exclusion chromatography wikipedia , lookup
Elementary particle wikipedia , lookup
Transition state theory wikipedia , lookup
Particle-size distribution wikipedia , lookup
Discodermolide wikipedia , lookup
Process chemistry wikipedia , lookup
Rate equation wikipedia , lookup
Implicit solvation wikipedia , lookup
George S. Hammond wikipedia , lookup
Atomic theory wikipedia , lookup
Thermometric titration wikipedia , lookup
Determination of equilibrium constants wikipedia , lookup
Geometrical frustration wikipedia , lookup
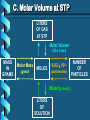
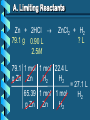
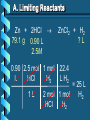
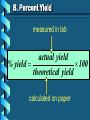
Stoichiometry – Ch. 9 I. Stoichiometric Calculations (p. 275-287) A. Proportional Relationships 2 1/4 c. flour 1 tsp. baking soda 1 tsp. salt 1 c. butter 3/4 c. sugar 3/4 c. brown sugar 1 tsp vanilla extract 2 eggs 2 c. chocolate chips Makes 5 dozen cookies. I have 5 eggs. How many cookies can I make? Ratio of eggs to cookies 5 eggs 5 doz. 2 eggs = 12.5 dozen cookies A. Proportional Relationships Stoichiometry • mass relationships between substances in a chemical reaction • based on the mole ratio Mole Ratio • indicated by coefficients in a balanced equation 2 Mg + O2 2 MgO B. Molar Volume at STP 1 mol of a gas=22.4 L at STP Standard Temperature & 0°C and 1 atm Pressure Note: When not at STP the ideal gas law will be used to convert between moles ↔ liters of gas (cover this later) C. Avogadro’s Number Number of particles in one mole of a substance. 1 mole = 6.022 x 1023 particles Can be used as a conversion factor between moles and Number of representative particles. Reminder: The representative particles for 1. an element is an atom 2. for a molecular compound is a molecule 3. for an ionic compound is a formula unit D. Molarity Molarity is a unit of concentration – it tells us the amount of solute in one liter of solution Molarity can be used to convert between moles and volume of solution. Molarity, M = moles of solute / liters of solution or Molarity, M = moles of solute / 1000 ml of solution E. Stoichiometry Steps 1. Write a balanced equation. 2. Label given and target. 3. Convert given unit to moles using the appropriate conversion factors. Conversion Factors: moles ↔ grams moles ↔ number of particles moles ↔ liters of solution moles ↔ liters of gas at STP Molar Mass Avogadro’s Number Molarity Standard Molar Volume 4. Convert moles of given substance to moles of target substance using the mole ration from the balanced equation. Note: This is the core step in all stoichiometry problems! 5. Convert moles of target to final unit using the appropriate conversion factor. C. Molar Volume at STP LITERS OF GAS AT STP Molar Volume (22.4 L/mol) MASS IN GRAMS Molar Mass (g/mol) 6.02 MOLES 1023 particles/mol Molarity (mol/L) LITERS OF SOLUTION NUMBER OF PARTICLES D. Stoichiometry Problems How many moles of KClO3 must decompose in order to produce 9 moles of oxygen gas? 2KClO3 2KCl + 3O2 ? mol 9 mol O2 2 mol KClO3 3 mol O2 9 mol = 6 mol KClO3 D. Stoichiometry Problems many grams of KClO3 are req’d to produce 9.00 L of O2 at STP? How 2KClO3 2KCl + 3O2 ?g 9.00 L 9.00 L O2 1 mol O2 2 mol 122.55 KClO3 g KClO3 22.4 L O2 3 mol O2 1 mol KClO3 = 32.8 g KClO3 D. Stoichiometry Problems How many grams of silver will be formed from 12.0 g copper? Cu + 2AgNO3 2Ag + Cu(NO3)2 12.0 g ?g 12.0 1 mol 2 mol 107.87 g Cu Cu Ag g Ag = 40.7 g 63.55 1 mol 1 mol Ag g Cu Cu Ag D. Stoichiometry Problems How many grams of Cu are required to react with 1.5 L of 0.10M AgNO3? Cu + 2AgNO3 2Ag + Cu(NO3)2 ?g 1.5L 0.10M 1.5 .10 mol 1 mol 63.55 L AgNO3 Cu g Cu 1L = 4.8 g 2 mol 1 mol Cu AgNO3 Cu Stoichiometry – Ch. 9 II. Stoichiometry in the Real World (p. 288-294) A. Limiting Reactants Available Ingredients • 4 slices of bread • 1 jar of peanut butter • 1/2 jar of jelly Limiting Reactant • bread Excess Reactants • peanut butter and jelly A. Limiting Reactants Limiting Reactant • used up in a reaction • determines the amount of product Excess Reactant • added to ensure that the other reactant is completely used up • cheaper & easier to recycle A. Limiting Reactants 1. Can be identified by the question involving amounts of more than one reactant. 2. For each reactant, calculate the amount of product formed using the 5 steps of stoichiometry. 3. Smaller answer indicates: • limiting reactant • amount of product A. Limiting Reactants Reminder - The five steps of stoichiometry 1. Write a balanced chemical equation. 2. Label your given and target substances. 3. Convert your given unit(s) to moles of given substance using the appropriate conversion factor. 4. Convert moles of given substance to moles of target substance using the mole ratio from the balanced equation. 5. Convert moles of target substance to the final unit using the appropriate conversion factor. Note: Carry out steps 3-5 as needed for both reactants in a limiting reactant problem. A. Limiting Reactants 79.1 g of zinc react with 0.90 L of 2.5M HCl. Identify the limiting and excess reactants. How many liters of hydrogen are formed at STP? Zn + 2HCl 79.1 g 0.90 L 2.5M ZnCl2 + H2 ?L A. Limiting Reactants Zn + 2HCl 79.1 g 0.90 L 2.5M ZnCl2 + H2 ?L 79.1 1 mol 1 mol 22.4 L g Zn Zn H2 H2 = 27.1 L 65.39 1 mol 1 mol H2 g Zn Zn H2 A. Limiting Reactants Zn + 2HCl 79.1 g 0.90 L 2.5M 0.90 2.5 mol 1 mol L HCl H2 1L ZnCl2 + H2 ?L 22.4 L H2 = 25 L 2 mol 1 mol H2 HCl H2 A. Limiting Reactants Zn: 27.1 L H2 HCl: 25 L H2 Limiting reactant: HCl Excess reactant: Zn Product Formed: 25 L H2 left over zinc B. Percent Yield measured in lab actual yield % yield 100 theoretical yield calculated on paper B. Percent Yield When 45.8 g of K2CO3 react with excess HCl, 46.3 g of KCl are formed. Calculate the theoretical and % yields of KCl. K2CO3 + 2HCl 2KCl + H2O + CO2 45.8 g ?g actual: 46.3 g B. Percent Yield K2CO3 + 2HCl 2KCl + H2O + CO2 45.8 g ?g actual: 46.3 g Theoretical Yield: 45.8 g 1 mol 2 mol 74.55 K2CO3 K2CO3 KCl g KCl = 49.4 138.21 g 1 mol 1 mol g KCl K2CO3 K2CO3 KCl B. Percent Yield K2CO3 + 2HCl 2KCl + H2O + CO2 45.8 g 49.4 g actual: 46.3 g Theoretical Yield = 49.4 g KCl % Yield = 46.3 g 49.4 g 100 = 93.7%