* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download ENTHALPY CHANGE DH
Resonance (chemistry) wikipedia , lookup
Stoichiometry wikipedia , lookup
Marcus theory wikipedia , lookup
Rutherford backscattering spectrometry wikipedia , lookup
Bioorthogonal chemistry wikipedia , lookup
Hypervalent molecule wikipedia , lookup
Chemical bond wikipedia , lookup
Thermomechanical analysis wikipedia , lookup
Chemical equilibrium wikipedia , lookup
Glass transition wikipedia , lookup
Electrochemistry wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Equilibrium chemistry wikipedia , lookup
Thermometric titration wikipedia , lookup
Spin crossover wikipedia , lookup
George S. Hammond wikipedia , lookup
Electrolysis of water wikipedia , lookup
Transition state theory wikipedia , lookup
Thermodynamics wikipedia , lookup
ENTHALPY CHANGE H
Enthalpy change is the heat energy change in a reaction measured under
conditions of constant pressure.
If the system gives out heat energy during a reaction, enthalpy is lost to the
surroundings, therefore the enthalpy change, H, has a negative value. This is an
exothermic reaction.
If the system takes in heat energy during a reaction, enthalpy is gained from the
surroundings, therefore the enthalpy change, H, has a positive value. This is an
endothermic reaction.
The size of an enthalpy change depends on the amount of substance used and on
the conditions of measurement. In order to make useful comparisons between
different measurements, standard amounts and standard conditions must be defined.
AMOUNT OF SUBSTANCE
The standard amount of substance is the mole. Thus an enthalpy change in a
reaction will be presented as, for example:
N2(g) + 3H2(g)
2NH3(g)
H = -92 kJ.mol-1
This means that when the molar quantities represented by the balanced equation
react, the heat given out by the reaction, at constant pressure, is 92 kJ.
H
=
Heat change at constant pressure
exothermic
92 kJ.
size of heat change
mol-1
for the molar quantities specified in the eqn.
STANDARD CONDITIONS
The size of the measured enthalpy change for a given quantity of reactant(s) in a
given reaction will depend also on the temperature and pressure at which it is
measured. Therefore, it is usual to quote enthalpy changes which have been
measured under agreed standard conditions.
Standard Pressure: The standard pressure chosen is 105 Pa (also called 1 bar).
Standard Temperature: The most common reference temperature is 298K.
Changes occurring under standard conditions are referred to as standard enthalpy
changes, indicated by the symbol Ho.
STANDARD STATES
Elements and compounds in their normal, stable state at 298K and 100kPa are said
to be in their standard state. The physical state is normally clarified further by the
inclusion of a state symbol. However, some elements can exist in allotropic forms.
TOPIC 13.17: THERMODYNAMICS
1
Allotropes are different forms of the same element capable of existing in the
same physical state, e.g. graphite and diamond.
Where allotropes exist, the particular allotrope should be identified in the balanced
equation; e.g. C (s, graphite) or C (s, diamond). If the allotrope is not specified, it is
assumed to be the more stable form under standard conditions: graphite in the case
of carbon.
STANDARD ENTHALPY CHANGES
The definitions of some standard enthalpy changes need to be
learned verbatim.
Standard Enthalpy of Formation, Hof
The standard enthalpy of formation is the enthalpy change when 1 mole of a
compound is formed from its elements under standard conditions, all reactants
and products being in their standard states.
e.g.
Na(s) + 1/2Cl2 (g)
NaCl(s)
Ca(s) + C(s,graphite) + 3/2O2(g)
CaCO3 (s)
By definition, the standard enthalpy of formation of an element must be
zero.
Many enthalpies of formation cannot be determined directly, because the reaction
concerned does not go, and they must instead be determined indirectly using
calculations based on Hess’s Law.
Standard Enthalpy of Combustion, Hoc
The standard enthalpy of combustion is the enthalpy change when 1 mole of a
substance is completely burned in oxygen under standard conditions, all
reactants and products being in their standard states.
e.g.
C (s) + O2 (g)
CH4 (g) + 2O2 (g)
CO2 (g)
H = -394 kJ.mol-1
CO2 (g) + 2H2O (l)
H = -890 kJ.mol-1
Ionisation Enthalpy, HoI
The molar first ionisation energy is the enthalpy change required to remove one
mole of electrons from one mole of gaseous atoms to form one mole of gaseous
positive ions.
e.g. K(g)
K+(g) + e- HI = +418kJ.mol-1
TOPIC 13.17: THERMODYNAMICS
2
Ionisation Enthalpy, HoI
The molar second ionisation energy is the enthalpy change required to remove
one mole of electrons from one mole of gaseous unipositive ions to form one mole
of gaseous dipositive ions.
e.g. K+(g)
K2+(g) + e- HI = +3070kJ.mol-1
The second ionisation enthalpy is always greater than the first, because the second
electron is being removed from a positive ion which is considerably smaller than the
atom. This requires more energy than removing an electron from a larger, neutral
species.
Electron Affinity, Hoea
The electron affinity is the enthalpy change when one mole of electrons is added
to one mole of gaseous atoms to form one mole of gaseous negative ions.
e.g. Cl(g) + e-
Cl-(g)
Hea = -364kJ.mol-1
The first electron affinity is usually exothermic.
Hea /kJ.mol-1
H(g)
F(g)
Cl(g)
Br(g)
I(g)
O(g)
O-(g)
- 72
-348
-364
-342
-314
-142
+844
The second electron affinity involves adding an electron to a negative ion. Owing to
the mutual repulsion of the ion and the electron, the enthalpy change is highly
endothermic.
The overall process of adding two electrons to a neutral atom is highly endothermic.
O(g) + 2eO2-(g)
H = + 702 kJ.mol-1
Bond Dissociation Enthalpy, Hodiss
The bond dissociation enthalpy is the standard molar enthalpy change which
accompanies the breaking of a covalent bond in a gaseous molecule to form two
gaseous free radicals.
e.g. H2(g)
2H(g) Hdiss = +436kJ.mol-1
CH4(g)
CH3(g) + H(g)Hdiss = +435kJ.mol-1
The species produced are free radicals and contain an unpaired electron. This can
be indicated by placing a dot after the formula, e.g. CH3.
The dot is usually omitted in thermodynamic equations.
TOPIC 13.17: THERMODYNAMICS
3
Bond Dissociation Enthalpies (kJ.mol-1)
H-H
N-H
C-Cl
Cl-Cl
C-H
C=O
C-O
436
391
338
242
412
805
358
C-C
O-H
H-Cl
H-Br
O=O
N N
348
463
431
366
496
945
Enthalpy of Atomisation, Hoat
The enthalpy of atomisation is the standard enthalpy change which accompanies
the formation of one mole of gaseous atoms from an element in its standard state.
e.g. 1/2H2(g)
Mg(s)
H(g)
Hat = +218 kJ.mol-1
Hat = +150 kJ.mol-1
Mg(g)
The relationship between the enthalpy of atomisation and other enthalpy changes
depends on the nature of the element:
C(s)
C(g)
Hat = +715 kJ.mol-1
Hat = Hsub
Hg(l)
Hg(g)
Hat = +61 kJ.mol-1
Hat = Hvap
Cl2(g)
Cl(g)
Hat = +121 kJ.mol-1
Hat = 1/2 Hdiss
Br2(l)
Br(g)
Hat = +112 kJ.mol-1
Hat = 1/2 Hvap + 1/2 Hdiss
Hat = +107 kJ.mol-1
Hat = 1/2 Hsub + 1/2 Hdiss
1/
2
1/
2
I2(s)
1/
2
I(g)
Enthalpy of Lattice Dissociation, Holatt
The enthalpy of lattice dissociation is the standard enthalpy change which
accompanies the separation of one mole of a solid ionic lattice into its gaseous
ions.
e.g. NaCl(s)
Na+(g) + Cl-(g)
Hlatt = +771 kJ.mol-1
Sometimes a lattice enthalpy is quoted as the enthalpy of lattice formation. This
involves the formation of a solid ionic lattice from its constituent gaseous ions and is
the reverse of the above process. Therefore the enthalpy change is exothermic
rather than endothermic but is equal in magnitude.
e.g. Na+(g) + Cl-(g)
TOPIC 13.17: THERMODYNAMICS
Hlatt = -771 kJ.mol-1
NaCl(s)
4
Born-Haber Cycles
The enthalpy of formation of an ionic solid can be broken down into a number of
other enthalpy changes, which can be arranged in a cycle known as a Born-Haber
cycle. One of the steps shown in the cycle is the enthalpy of lattice dissociation; this
change cannot be measured directly but can be deduced from the cycle by the
application of Hess’s Law. The cycle for sodium chloride is shown below.
Na+(g) + e- + Cl(g)
Hat = +121
enthalpy of atomisation of Cl
Hea = -364
Na+(g) + e- + 1/2Cl2(g)
Electron affinity
of Cl
Na+(g) + Cl-(g)
1st ionisation enthalpy of Na
Energy
kJ.mol-1
Na(g) +
1/
HI = +494
Hlatt = +771 enthalpy of lattice
dissociation of NaCl
2Cl2(g)
enthalpy of atomisation of Na
Hat = +109
Na(s) + 1/2Cl2(g)
enthalpy of formation
of NaCl
Hf = -411
NaCl(s)
Applying Hess’s Law:
Hf = Hat + HI + Hat + Hea - Hlatt
hence
Hlatt = Hat + HI + Hat + Hea - Hf
Hlatt = + 109 + 494 + 121 + (-364) - (-411)
Hlatt = + 771 kJ.mol-1
TOPIC 13.17: THERMODYNAMICS
5
Deviation from the ionic model
The IONIC MODEL can be used to calculate theoretical lattice enthalpies. This
model assumes that ions are spherical in shape and that the charge on the ion is
evenly distributed.
The values of lattice enthalpy found using the Ionic Model and those found
experimentally using a Born-Haber cycle often differ significantly.
Compound
NaCl
NaBr
NaI
Hlatt (experimental)
-787
-742
-698
Hlatt (theory)
-766
-731
-686
In the table above the theoretical and experimental values agree very well suggesting
that the bonding present in the sodium halides is essentially ionic.
However, the agreement between the magnesium halides is not so good.
Compound
MgCl2
MgBr2
MgI2
Hlatt (experimental)
-2526
-2440
-2327
Hlatt (theory)
-2326
-2097
-1944
The experimental (real) values are some 10-15% higher than the theoretical values.
This suggests that the bonding involved is stronger than the ionic model predicts.
The difference is that, due to the small and more highly charged nature of the
magnesium ion, the halide ions have been polarised (and hence their shape
distorted) and there is additional degree of covalent character within bonding. The
smaller and more highly charged the metal ion the greater the deviation from the
ionic model.
TOPIC 13.17: THERMODYNAMICS
6
ENTHALPY OF SOLUTION
Enthalpy of Solution, Hosol
The enthalpy of solution is the standard enthalpy change for the process in which
one mole of an ionic solid dissolves in an amount of water large enough to ensure
that the dissolved ions are well separated and do not interact with one another.
Na+(aq) + Cl-(aq) Hsol = +2 kJ.mol-1
e.g. NaCl(s) + aq.
When an ionic solid dissolves in water, the process can be broken down into two
separate processes:
the breaking down of the solid lattice into gaseous ions, which will require the
enthalpy of lattice dissociation
e.g.
NaCl(s)
Na+(g) + Cl-(g)
the hydration of the gaseous ions, which will release energy equivalent to the
enthalpy of hydration
e.g.
Na+(g) + Cl-(g) + aq.
Na+(aq) + Cl-(aq)
Enthalpy of Hydration, Hohyd
The enthalpy of hydration is the standard molar enthalpy change for the process:
e.g.
+
X - (g)
+
X- (aq)
The symbol (+/-) is used to indicate either a cation or an anion.
The energy diagram below illustrates the enthalpy of solution of sodium chloride.
Na+(g) + Cl-(g) + aq.
enthalpy of hydration of Na+
Energy
kJ.mol-1
Hhyd = -405
enthalpy of lattice
dissociation of NaCl
Na+(aq) + Cl-(g) + aq.
enthalpy of hydration of ClHhyd = -364
Na+(aq) + Cl-(aq)
Hlatt = +771
NaCl(s) + aq.
TOPIC 13.17: THERMODYNAMICS
7
Hsol = +2
Calculation of the standard enthalpy change for a reaction from
standard enthalpies of formation Hof
In general, for any reaction:
Ho = Hof(products) - Hof(reactants)
Example: Calculate the standard enthalpy change for the reaction:
given that:
Fe2O3(s) + 3CO(g)
2Fe(s) + 3CO2(g)
Hof (Fe2O3) = - 822 kJ.mol-1
Hof (CO)
= - 111 kJ.mol-1
Hof (CO2) = - 394 kJ.mol-1
Ho = Hof(products) - Hof(reactants)
Ho = {(-394 x 3) + 0)} - {(-111 x 3) + (-822)}
Ho = - 27 kJ.mol-1
TOPIC 13.17: THERMODYNAMICS
8
BOND ENTHALPY
The bond dissociation enthalpy of a diatomic molecule refers to the enthalpy
change for the process:
A
B(g)
A(g) + B(g)
Note that all the species are in the gaseous state.
In polyatomic molecules (those containing 3 or more atoms), it is usual to use mean
bond enthalpy.
Mean bond enthalpy is the energy required to break a particular covalent bond,
averaged over a large number of compounds.
Consider the following examples, which show that the energy required to break a
particular bond depends on its specific environment.
H2O(g)
OH(g)
OH(g) + H(g)
O(g) + H(g)
H = +495 kJ.mol-1
H = +428 kJ.mol-1
The mean bond enthalpy of the O-H bond is +463 kJ.mol-1
CH4(g)
CH4(g)
CH3(g) + H(g)
C(g) + 4H(g)
H = +423 kJ.mol-1
H = +1664 kJ.mol-1
The mean bond enthalpy of the C-H bond is +412 kJ.mol-1
Example: Use the data given below to calculate a value for the bond
dissociation enthalpy of the C-H bond.
Hof (CH4)
Hoat(C)
Hodiss(H2)
CH4(g)
Hof
= - 74 kJ.mol-1
= +720 kJ.mol-1
= +431 kJ.mol-1
Ho
Hoat
C(g) + 4H(g)
2 x Hodiss
C(s) + 2H2(g)
By Hess’s Law:
Ho = -Hof + Hoat + 2 x Hodiss
= - (-74) + (+720) + (+431 x 2)
= + 1656 kJ.mol-1
Ho = 4 x Hdiss (C-H)
Hdiss (C-H) = +1656 = + 414 kJ.mol-1
4
TOPIC 13.17: THERMODYNAMICS
9
CALCULATION OF H FROM MEAN BOND ENTHALPIES
When a chemical change takes place, existing chemical bonds are broken and new
bonds are formed. Bond breaking requires an input of energy, therefore the process
is endothermic. The formation of a bond releases energy and is therefore exothermic.
The overall enthalpy change in the reaction will be the difference between the energy
required for the endothermic step and the energy released by the exothermic step.
Mean bond enthalpies can be used to calculate the enthalpy change in simple
reactions. Since mean bond enthalpies are used, the value obtained is only an
approximation.
H = Hdiss(bonds broken) – Hdiss(bonds formed)
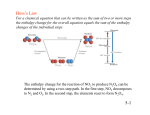
Example: Calculate the enthalpy change in the reaction:
N2(g) + 3H2(g)
2NH3(g)
Bonds broken
1x N N
3 x H-H
Bonds formed
6 x N-H
Hdiss(N N) = +945 kJ.mol-1
Hdiss(H-H) = +436 kJ.mol-1
Hdiss(N-H) = +391 kJ.mol-1
H = (+945) + (+436 x 3) - (+391 x 6)
= - 93 kJ.mol-1
Bond enthalpy calculations apply only to reactions carried out in the gaseous
state.
TOPIC 13.17: THERMODYNAMICS
10
FREE ENERGY & SPONTANEOUS CHANGE
A spontaneous change is one which has a natural tendency to occur if left to itself.
For example:
the water in a lake will freeze spontaneously if the air temperature above it is
below 0oC.
ice cubes placed in a drink will melt spontaneously
iron in the presence of oxygen and water will rust spontaneously
a battery will spontaneously run down
However, the spontaneous change will only occur in one direction unless conditions
are changed. It is often difficult to reverse a spontaneous change.
the water in the lake will not melt unless the temperature of the air above it
rises
water needs the electrical energy used by a fridge to convert it into ice cubes
iron oxide needs the conditions produced in a blast furnace to turn it back to
iron
a battery can be re-charged using electrical energy from the mains
The quantity which decides whether a particular change can occur spontaneously is
the free-energy change, which is given the symbol G. For a change to occur
spontaneously:
.
G must be negative or zero
Free energy changes combine the effects, in a particular reaction, of changes in
enthalpy and changes in entropy.
G = H - TS
You will notice that the above equation takes the form of y=mx+c (the equation of a
straight line). A plot of ∆G against T (in K) gives a straight line graph with intercept
∆H and gradient -∆s.
50
40
∆G (kJ/mol)
30
20
10
0
-10 0
200
400
800
1000
Temperature (K)
-20
-30
-40
TOPIC 13.17: THERMODYNAMICS
600
11
1. Changes in Enthalpy
The sign of an enthalpy change does not allow a prediction to be made about the
feasibility of a change.
There is a natural tendency for enthalpy to fall in a reaction, and many exothermic
changes occur spontaneously. For example, the reaction between potassium and
water occurs spontaneously and liberates a lot of heat:
2K(s) + 2H2O(l)
2KOH(aq) + H2(g)
However, spontaneous endothermic changes are also known, for example the
reaction of sodium hydrogencarbonate with dilute hydrochloric acid:
NaHCO3(s) + HCl(aq)
NaCl(aq) + H2O(l) + CO2(g)
The fact that endothermic changes can occur spontaneously clearly indicates that a
factor other than enthalpy change must also be involved. This additional factor is the
change in entropy.
TOPIC 13.17: THERMODYNAMICS
12
2. Entropy
Entropy can best be thought of as a measure of the disorder of a chemical system;
the more disordered the system, the higher the entropy. There is a natural tendency
for the entropy of a system to increase.
The particles in a solid are ordered and their movement is restricted to vibration
about a mean position. The particles in a gas are disordered and they move about
randomly. Therefore, the entropy of a gas is much higher than that of a solid.
entropy increases
solid
liquid
gas
ordered
less ordered
chaotic
When a solid is heated, the amplitude of the vibrating particles increases, and
therefore the entropy increases. When the solid reaches its melting point, the entropy
increases at constant temperature as the ordered solid becomes a less ordered
liquid.
When the liquid is heated, the kinetic energy of the particles increases, and therefore
the entropy increases. When the liquid reaches its boiling point, the entropy
increases at constant temperature as the less ordered liquid becomes a chaotic gas.
The entropy change from liquid to gas is greater than that from solid to liquid.
entropy
gas
liquid
solid
m.p
.
b.p.
temperature
TOPIC 13.17: THERMODYNAMICS
13
The units of entropy are:
J.K-1.mol-1
Entropy decreases as temperature decreases, so that at absolute zero (0K), most
substances are solids consisting of perfectly ordered particles which have ceased to
vibrate. They therefore have zero entropy. This means that there is a definite starting
point for entropy, and substances can be assigned an absolute standard entropy
vaue (So). Compare this with enthalpy, where only changes can be measured, and
where it is necessary to assign to elements the arbitrary standard enthalpy of zero.
The table below lists some standard entropies.
So / J.K-1.mol-1
CH4(g)
C(s,diamond)
C(s,graphite)
Na2CO3(s)
Cl2(g)
O2(g)
H2O(g)
C2H5OH(l)
C6H12(l)
186
2.4
5.7
136
223
205
189
161
204
H2O(l)
NaCl(s)
NaHCO3(s)
Mg(s)
H2(g)
CO2(g)
CH3OH(l)
C6H6(l)
69.9
72.4
102
32.5
131
214
127
173
2. Changes in Entropy
a) Chemical Changes
The entropy change during a chemical reaction is calculated from the expression:
So = So(products) - So(reactants)
Example: Calculate the standard entropy change for the combustion of methane:
CH4(g) + 2O2(g)
CO2(g) + 2H2O(l)
So =
o(products)
o(products)
- So(reactants)
= 214 + 2 x 69.9 = 353.8 J.K-1.mol-1
So(reactants) = 186 + 2 x 205 = 596 J.K-1.mol-1
So = 353.8 - 596 = -242.2 J.K-1.mol-1
There is a fall in the entropy of the system (i.e. it becomes more ordered),
because three moles of gas react to form one mole of gas and two moles of liquid.
Liquids are more ordered than gases.
TOPIC 13.17: THERMODYNAMICS
14
b) Physical Changes
When a system is in equilibrium between two physical states, for example water
and steam at 373K and 105Pa, there is no spontaneous tendency for the equilibrium
mixture to change. Unless the external conditions are changed, the mixture will
neither spontaneously liquefy nor vaporise. Since there is no tendency to move
spontaneously in either direction, the free energy change, G is zero.
Since G = H - TS, when G = 0
H = TS
S = H
T
Consider water boiling in a kettle. We can calculate the change in entropy (S) when
water boils as follows.
Say a 2.4kW kettle (which delivers 2.4kJ of energy per second) turns 100g of water
to steam in 100s.
Calculate the enthalpy change of vaporisation (Hvap) when one mole of water (at
100oC) is vaporised.
Heat energy delivered by kettle in 100s = 2.4 x 100
= 240 kJ
Moles of water vaporised = mass/Mr = 100/18 = 5.55
Hence enthalpy of vaporisation = 240/5.55 = 43.2 kJ/mol
Since this happens at 373K
Svap = Hvap = 43.2 x 1000 = +115.8 J.K-1.mol-1
Tb
373
Nb Hvap has been converted into J.mol-1 (x1000) in order that s be
calculated in the normal entropy units of J.K-1.mol-1
TOPIC 13.17: THERMODYNAMICS
15
FREE ENERGY CHANGES
1. H is negative; S is positive
+
Energy
change
Temperature /K
0
H
-
TS
G
Since G is always negative, a change of this type is feasible at any temperature.
Examples:
NH4NO3(s)
2H2O(l) + N2O(g)
C(s) + O2(g)
CO2(g)
2. H is positive; S is positive
H
+
change feasible
Temperature /K
Energy
change
0
change not feasible
-
G
TS
G becomes zero where the line crosses the x-axis (temperature). At temperatures
above this point, G is negative and the change is feasible. Below this temperature,
G is positve and the change is not feasible.
Examples:
H2O(l)
H2O(g)
CaCO3(s)
boiling
CaO(s) + CO2(g)
NaHCO3(s) + HCl(aq)
TOPIC 13.17: THERMODYNAMICS
NaCl(aq) + H2O(l) + CO2(g)
16
3. H is negative; S is negative
TS
+
Energy
change
change not feasible
0
G
Temperature /K
-
change feasible
H
G becomes zero where the line crosses the x-axis (temperature). At temperatures
below this point, G is negative and the change is feasible. Above this temperature,
G is positve and the change is not feasible.
Examples:
H2O(g)
H2O(l)
N2(g) + 3H2(g)
condensation
2NH3(g)
4. H is positive; S is negative
G
TS
H
+
Energy
change
0
Temperature /K
-
G is always positive, so a change of this type is never feasible.
TOPIC 13.17: THERMODYNAMICS
17
Calculating the temperature at which a reaction becomes
feasible.
Referring to the diagrams on the previous two pages, a change just becomes
feasible when G = 0.
Since G = H - TS, when G = 0
H = TS
T = H
S
It is important in this calculation to ensure that H and S are in compatible
units. H is usually given in kJ.mol-1, therefore, S must be converted to kJ.K-1.mol-1
by dividing by 1000. The answer will be in K.
Example: Use the data below to calculate the temperature at which the following
reaction becomes feasible.
CaCO3(s)
CaO(s) + CO2(g)
Hf /kJ.mol-1
CaCO3(s)
-1207
CaO(s)
-635
CO2(g)
-394
S /J.K-1.mol-1
88.7
40
214
Ho = o(products) - Ho(reactants)
= (-635-394) – (-1207)
= + 178 KJ.mol-1
So = o(products) - So(reactants)
= (40 + 214) – (88.7)
= + 165.3 J.K-1.mol-1
Go = Ho - TSo
when the reaction just becomes feasible, G = 0
The temperature at which this occurs is given by T = Ho
So
T = 178 x 1000 = 1077K
165.3
TOPIC 13.17: THERMODYNAMICS
18