AP Chemistry:
... 0.50 M NaOH and 0.50 M HCl solutions. Which of the following situations, by itself, would most likely result in the LEAST error in the calculated value of the heat of reaction? (A) The thermometer was incorrectly calibrated and read 0.5 Celsius degree too high during the procedure. (B) The volume of ...
... 0.50 M NaOH and 0.50 M HCl solutions. Which of the following situations, by itself, would most likely result in the LEAST error in the calculated value of the heat of reaction? (A) The thermometer was incorrectly calibrated and read 0.5 Celsius degree too high during the procedure. (B) The volume of ...
CHE 1402 Lab Manual
... Place 0.30 g of CaCO3 in a test tube and carefully insert another smaller test tube in it containing 5mL of 4 M HCl (be sure NOT to mix CaCO3 and HCl before the experiment). Assemble the apparatus illustrated in Figure 2.1 but do not attach the test tube. Be sure that tube B does not extend below th ...
... Place 0.30 g of CaCO3 in a test tube and carefully insert another smaller test tube in it containing 5mL of 4 M HCl (be sure NOT to mix CaCO3 and HCl before the experiment). Assemble the apparatus illustrated in Figure 2.1 but do not attach the test tube. Be sure that tube B does not extend below th ...
Use the following answers for questions 1
... (E) Volume of water displaced from the flask 78. When the actual gas volume is greater then the volume predicted by the ideal gas law, the explanation lies in the fact that the ideal gas law does NOT include a factor for molecular. (A) volume (B) mass (C) velocity (D) attractions (E) shape 85. A sam ...
... (E) Volume of water displaced from the flask 78. When the actual gas volume is greater then the volume predicted by the ideal gas law, the explanation lies in the fact that the ideal gas law does NOT include a factor for molecular. (A) volume (B) mass (C) velocity (D) attractions (E) shape 85. A sam ...
2(g)
... Excess and limiting reagents refer to the reactant that will run out first and stop more product from forming. ...
... Excess and limiting reagents refer to the reactant that will run out first and stop more product from forming. ...
class notes 4
... Have them switch partners and write the equations correctly based on charges. However H2CO3(aq) decomposes to water and carbon dioxide. Thus, the equation: 2 HNO3(aq) + Na2CO3(aq) H2O(l) + CO2(g) + 2 NaNO3(aq) Finish by balancing the equations with the correct coefficients. ...
... Have them switch partners and write the equations correctly based on charges. However H2CO3(aq) decomposes to water and carbon dioxide. Thus, the equation: 2 HNO3(aq) + Na2CO3(aq) H2O(l) + CO2(g) + 2 NaNO3(aq) Finish by balancing the equations with the correct coefficients. ...
National 5 - Deans Community High School
... (iii) Explain why your answer to (ii) is not half your answer to (i). (c) Calculate the average rate of reaction over the first 25 s. ...
... (iii) Explain why your answer to (ii) is not half your answer to (i). (c) Calculate the average rate of reaction over the first 25 s. ...
Test 1 Pre test
... Which one of the following statements is false? For a reaction carried out at constant temperature and constant pressure in an open container, ____. a. the work done by the system can be set equal to PV b. the work done by the system can be set equal to VP c. the work done by the system can be se ...
... Which one of the following statements is false? For a reaction carried out at constant temperature and constant pressure in an open container, ____. a. the work done by the system can be set equal to PV b. the work done by the system can be set equal to VP c. the work done by the system can be se ...
Chemical Equilibrium - Chemistry Teaching Resources
... The strong base will have dissociated completely meaning that all the OH—(aq) ions were available to react with the Fe3+(aq) ions . The weak base is only partially dissociated so less than 1% of the OH—(aq) ions are available at the beginning. However, as the OH—(aq) ions react with Fe3+(aq) they ...
... The strong base will have dissociated completely meaning that all the OH—(aq) ions were available to react with the Fe3+(aq) ions . The weak base is only partially dissociated so less than 1% of the OH—(aq) ions are available at the beginning. However, as the OH—(aq) ions react with Fe3+(aq) they ...
Exam 980415 - NTOU-Chem
... C) a Lewis base. D) a Bronsted-Lowry base. E) insoluble in the solvent. Answer: A 42) For the phase transition from solid to liquid, ...
... C) a Lewis base. D) a Bronsted-Lowry base. E) insoluble in the solvent. Answer: A 42) For the phase transition from solid to liquid, ...
15.0 EquilibriumIHS2014
... increasing the container volume. Then the equilibrium shifts to the left (the side with more moles of gas) • At B, the temperature is increased. Then the equilibrium shifts to left. • At C, C2H6(g) is added to the system. Then the equilibrium shifts to the left. • At D, no shift in equilibrium posit ...
... increasing the container volume. Then the equilibrium shifts to the left (the side with more moles of gas) • At B, the temperature is increased. Then the equilibrium shifts to left. • At C, C2H6(g) is added to the system. Then the equilibrium shifts to the left. • At D, no shift in equilibrium posit ...
chemistry sp.indd
... Permission to reproduce items where third-party owned material protected by copyright is included has been sought and cleared where possible. Every reasonable effort has been made by the publisher (UCLES) to trace copyright holders, but if any items requiring clearance have unwittingly been included ...
... Permission to reproduce items where third-party owned material protected by copyright is included has been sought and cleared where possible. Every reasonable effort has been made by the publisher (UCLES) to trace copyright holders, but if any items requiring clearance have unwittingly been included ...
Last Name Professor BEAMER First Name
... Solution/Explanation: You are converting between particles (molecules) and mass (grams). Therefore, you need to use Avogadro’s Number. ...
... Solution/Explanation: You are converting between particles (molecules) and mass (grams). Therefore, you need to use Avogadro’s Number. ...
Topic 5 Energetics File
... Entropy: A measure of the disorder of a system. Things causing entropy to increase: 1) increase of number of moles of gaseous molecules; 2) change of state from solid to liquid or liquid to gas; 3) increase of temperature Exothermic: A reaction in which energy is evolved. ΔH is –. Products more stab ...
... Entropy: A measure of the disorder of a system. Things causing entropy to increase: 1) increase of number of moles of gaseous molecules; 2) change of state from solid to liquid or liquid to gas; 3) increase of temperature Exothermic: A reaction in which energy is evolved. ΔH is –. Products more stab ...
Thermochemistry Chem 2/H
... 450mL of 2M HCl are mixed with 450mL of 2M NaOH in a coffee-cup calorimeter. The temperature rises from 23oC to 25oC. Calculate the enthalpy change for this reaction. ...
... 450mL of 2M HCl are mixed with 450mL of 2M NaOH in a coffee-cup calorimeter. The temperature rises from 23oC to 25oC. Calculate the enthalpy change for this reaction. ...
Keq = [A] [B] [C] [D]
... If we know the amounts or concentrations of the reactants and products of a chemical system at equilibrium, we can calculate the value of the equilibrium constant Keq. Similarly, if we know the value of Keq at a specified temperature, and the initial concentrations of the reactants, we can calculate ...
... If we know the amounts or concentrations of the reactants and products of a chemical system at equilibrium, we can calculate the value of the equilibrium constant Keq. Similarly, if we know the value of Keq at a specified temperature, and the initial concentrations of the reactants, we can calculate ...
Chem 2A Final Review
... ------------------------------ Potentially Useful Information for the Problems Below-----------------------------R=0.0821 (atm L)/(mol K) -------------------------------------------------------------------------------------------------1. An acidic solution has a pH of 3.43. What is the Hydronium ion ...
... ------------------------------ Potentially Useful Information for the Problems Below-----------------------------R=0.0821 (atm L)/(mol K) -------------------------------------------------------------------------------------------------1. An acidic solution has a pH of 3.43. What is the Hydronium ion ...
Stoichiometry of Chemical Reactions
... common patterns of behavior. When two humans exchange information, we say they are communicating. When they exchange blows with their fists or feet, we say they are fighting. Faced with a wide range of varied interactions between chemical substances, scientists have likewise found it convenient (or ...
... common patterns of behavior. When two humans exchange information, we say they are communicating. When they exchange blows with their fists or feet, we say they are fighting. Faced with a wide range of varied interactions between chemical substances, scientists have likewise found it convenient (or ...
Stoichiometry of Chemical Reactions
... common patterns of behavior. When two humans exchange information, we say they are communicating. When they exchange blows with their fists or feet, we say they are fighting. Faced with a wide range of varied interactions between chemical substances, scientists have likewise found it convenient (or ...
... common patterns of behavior. When two humans exchange information, we say they are communicating. When they exchange blows with their fists or feet, we say they are fighting. Faced with a wide range of varied interactions between chemical substances, scientists have likewise found it convenient (or ...
1. What energy changes occur when chemical bonds are formed
... The reaction is spontaneous at low temperatures but becomes non-spontaneous at high temperatures. ...
... The reaction is spontaneous at low temperatures but becomes non-spontaneous at high temperatures. ...
chapter 21 chemistry of the main-group elements i
... MgF2 vs. MgCl2 solubility in water. F is smaller than Cl, hence, electrostatic attraction between Mg2+ and the halide is greater in F than Cl. If we assume that hydration of the ions is similar, we expect that MgF2 is less soluble than MgCl2. (Note: Ksp given for MgF2, which is sparingly soluble ...
... MgF2 vs. MgCl2 solubility in water. F is smaller than Cl, hence, electrostatic attraction between Mg2+ and the halide is greater in F than Cl. If we assume that hydration of the ions is similar, we expect that MgF2 is less soluble than MgCl2. (Note: Ksp given for MgF2, which is sparingly soluble ...
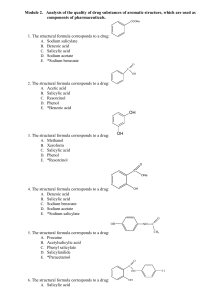
Module 2. Drug substances of aromatic structure
... 54. The initial substance for synthesis of benzoic acid is: A. Pyrocatechol B. Benzoic acid C. Salicylic acid D. Phenol E. *Toluene 55. The chemical name of sodium salicylate: A. Sodium benzenecarboxylate B. Benzenecarboxylic acid C. 2-Hydroxybenzenecarboxylic acid D. Oxybenzene E. *2-Hydroxybenzene ...
... 54. The initial substance for synthesis of benzoic acid is: A. Pyrocatechol B. Benzoic acid C. Salicylic acid D. Phenol E. *Toluene 55. The chemical name of sodium salicylate: A. Sodium benzenecarboxylate B. Benzenecarboxylic acid C. 2-Hydroxybenzenecarboxylic acid D. Oxybenzene E. *2-Hydroxybenzene ...
Chem 107 - Hughbanks Exam 1
... (3) (7 points) Calcium carbide, CaC2, is an important preliminary chemical for industries producing synthetic fabrics and plastics. CaC2 may be produced by heating calcium oxide with coke (a source of carbon). CaC2 is be reacted with nitrogen, N2 at 1000 – 1200 ˚C to produce another important chemic ...
... (3) (7 points) Calcium carbide, CaC2, is an important preliminary chemical for industries producing synthetic fabrics and plastics. CaC2 may be produced by heating calcium oxide with coke (a source of carbon). CaC2 is be reacted with nitrogen, N2 at 1000 – 1200 ˚C to produce another important chemic ...
Energy and Chemistry
... like pressure, volume, and temperature affect the energy content of a system. What we need is a definition of energy that holds when some of these conditions are specified (somewhat similar to our definition of standard temperature and pressure in our study of gases). We define the enthalpy change (ΔH) a ...
... like pressure, volume, and temperature affect the energy content of a system. What we need is a definition of energy that holds when some of these conditions are specified (somewhat similar to our definition of standard temperature and pressure in our study of gases). We define the enthalpy change (ΔH) a ...
Properties of Systems in Equilibrium - Le
... mixture. In other words you will be able to determine whether the equilibrium position lies to the left (more reactants and less products) or whether the equilibrium lies to the right (more products and less reactants). Your goal will be to find a reagent that will shift the position of this equilib ...
... mixture. In other words you will be able to determine whether the equilibrium position lies to the left (more reactants and less products) or whether the equilibrium lies to the right (more products and less reactants). Your goal will be to find a reagent that will shift the position of this equilib ...
1aUnit Two Handouts - Dunmore High School
... 15. Magnesium turnings are added to a solution of iron (III) chloride. ...
... 15. Magnesium turnings are added to a solution of iron (III) chloride. ...
Thermometric titration

A thermometric titration is one of a number of instrumental titration techniques where endpoints can be located accurately and precisely without a subjective interpretation on the part of the analyst as to their location. Enthalpy change is arguably the most fundamental and universal property of chemical reactions, so the observation of temperature change is a natural choice in monitoring their progress. It is not a new technique, with possibly the first recognizable thermometric titration method reported early in the 20th century (Bell and Cowell, 1913). In spite of its attractive features, and in spite of the considerable research that has been conducted in the field and a large body of applications that have been developed; it has been until now an under-utilized technique in the critical area of industrial process and quality control. Automated potentiometric titration systems have pre-dominated in this area since the 1970s. With the advent of cheap computers able to handle the powerful thermometric titration software, development has now reached the stage where easy to use automated thermometric titration systems can in many cases offer a superior alternative to potentiometric titrimetry.The applications of thermometric titrimetry discussed on this page are by no means exhaustive. The reader is referred to the bibliography for further reading on the subject.













![Keq = [A] [B] [C] [D]](http://s1.studyres.com/store/data/014463360_1-50a2de0db1e8b9a361c4b31c6e85c28d-300x300.png)