Cathode ray deflection tube
... A bar magnet can now be held at the side of the tube and you will see that the beam of electrons is deflected up or down depending which way round you hold the magnet. The same thing will happen of course if you use an electromagnet (see Figure 3). Magnetic field at right angles to the paper ...
... A bar magnet can now be held at the side of the tube and you will see that the beam of electrons is deflected up or down depending which way round you hold the magnet. The same thing will happen of course if you use an electromagnet (see Figure 3). Magnetic field at right angles to the paper ...
Quantum-Electrodynamics and the Magnetic Moment of the
... The new Hamiltonian is superior to the original one in essentially three ways: it involves the experimental electron mass, rather than the unobservable mechanical mass; an electron now interacts with the radiation field only in the presence of an external field, that is, only an accelerated electron ...
... The new Hamiltonian is superior to the original one in essentially three ways: it involves the experimental electron mass, rather than the unobservable mechanical mass; an electron now interacts with the radiation field only in the presence of an external field, that is, only an accelerated electron ...
AP Chemistry Study Guide – Chapter 7, Atomic Structure
... 6) Account for each of the following in terms of principles of atomic structure, including the number, properties, and arrangements of subatomic particles. (a) The second ionization energy of sodium is about three times greater than the second ionization energy of magnesium. (b) The difference betwe ...
... 6) Account for each of the following in terms of principles of atomic structure, including the number, properties, and arrangements of subatomic particles. (a) The second ionization energy of sodium is about three times greater than the second ionization energy of magnesium. (b) The difference betwe ...
Electronic Magnetic Moments
... -An orbital magnetic moment due to orbital angular momentum -A spin magnetic moment due to electron spin ...
... -An orbital magnetic moment due to orbital angular momentum -A spin magnetic moment due to electron spin ...
mse seminar - Virginia Tech
... “Spin and Quantum-coherent Electron Transport in Semiconductors” ABSTRACT Spin-dependent electronic properties in semiconductor structures may be utilized toward the design of novel spintronics implementations, and also form a starting point to explore captivating physical phenomena. In particular, ...
... “Spin and Quantum-coherent Electron Transport in Semiconductors” ABSTRACT Spin-dependent electronic properties in semiconductor structures may be utilized toward the design of novel spintronics implementations, and also form a starting point to explore captivating physical phenomena. In particular, ...
Slide 1
... – The intensity at different angles hints to structure of atoms. • WHY? – Investigate the internal structure of particles – To understand early methods of determining properties – Scattering (fixed target experiment) is a method to do particle physics (particle production, detection …) ...
... – The intensity at different angles hints to structure of atoms. • WHY? – Investigate the internal structure of particles – To understand early methods of determining properties – Scattering (fixed target experiment) is a method to do particle physics (particle production, detection …) ...
Snímek 1 - Cesta k vědě - Gymnázium Jaroslava Seiferta
... Introduction Nuclear magnetic resonance (NMR) is a physical phenomenon based upon the quantum mechanical magnetic properties of an atom's nucleus. All nuclei that contain odd numbers of protons or neutrons have an intrinsic magnetic moment and angular momentum. The most commonly measured nuclei are ...
... Introduction Nuclear magnetic resonance (NMR) is a physical phenomenon based upon the quantum mechanical magnetic properties of an atom's nucleus. All nuclei that contain odd numbers of protons or neutrons have an intrinsic magnetic moment and angular momentum. The most commonly measured nuclei are ...
PH4042 - Concepts in Atomic Physics and Magnetic Resonance
... This module builds on the atomic physics covered in PH4041 to look at the atomic structure of helium and many-electron atoms, magnetic interactions within the atom (leading to fine and hyperfine splitting), the Zeeman effect, and topics in atom-light interaction. These well-established concepts are ...
... This module builds on the atomic physics covered in PH4041 to look at the atomic structure of helium and many-electron atoms, magnetic interactions within the atom (leading to fine and hyperfine splitting), the Zeeman effect, and topics in atom-light interaction. These well-established concepts are ...
Nuclear Magnetic Resonance, NMR
... field over a small range while observing the rf signal from the sample. An equally effective technique is to vary the frequency of the rf radiation while holding the external field constant. ...
... field over a small range while observing the rf signal from the sample. An equally effective technique is to vary the frequency of the rf radiation while holding the external field constant. ...
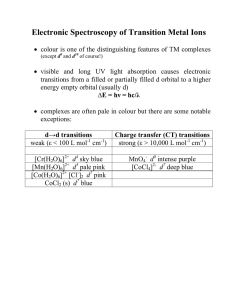
Electronic Spectroscopy of Transition Metal Ions
... 2nd electron: can go in any d orbital but only spin paired in the one already occupied = 9 possibilities • therefore there are 90 ways to do this but since e- are indistinguishable, there are actually only 45 unique arrangements (called ‘microstates’ of the system) • but L and S are quantized so an ...
... 2nd electron: can go in any d orbital but only spin paired in the one already occupied = 9 possibilities • therefore there are 90 ways to do this but since e- are indistinguishable, there are actually only 45 unique arrangements (called ‘microstates’ of the system) • but L and S are quantized so an ...
Physical and Chemical Tests
... A 1H nucleus is positively charged and its spinning motion generates a magnetic field. In the presence of an external magnetic field, H0, the magnetic field of the hydrogen nucleus can be oriented either with H0 (lower energy) or against H0 (higher energy). These two states are called and spin s ...
... A 1H nucleus is positively charged and its spinning motion generates a magnetic field. In the presence of an external magnetic field, H0, the magnetic field of the hydrogen nucleus can be oriented either with H0 (lower energy) or against H0 (higher energy). These two states are called and spin s ...
15 2.1 Introduction: This chapter discuss briefly about the EPR
... structure of the host lattices, and interpretation of powder and single crystal EPR spectra. The procedure for the calculation of the direction cosines of the metal - ligand directions from single crystal data is also mentioned. Spin-Hamiltonian parameters from single crystal EPR spectra and Simulat ...
... structure of the host lattices, and interpretation of powder and single crystal EPR spectra. The procedure for the calculation of the direction cosines of the metal - ligand directions from single crystal data is also mentioned. Spin-Hamiltonian parameters from single crystal EPR spectra and Simulat ...
Spin Angular Momentum Magnetic Moments
... can be approximated to the behaviour of a classical rotor such as a gyroscope or bicycle wheel and the mathematics associated with these types of systems are applicable to many NMR systems. ...
... can be approximated to the behaviour of a classical rotor such as a gyroscope or bicycle wheel and the mathematics associated with these types of systems are applicable to many NMR systems. ...
Electron paramagnetic resonance
Electron paramagnetic resonance (EPR) or electron spin resonance (ESR) spectroscopy is a technique for studying materials with unpaired electrons. The basic concepts of EPR are analogous to those of nuclear magnetic resonance (NMR), but it is electron spins that are excited instead of the spins of atomic nuclei. EPR spectroscopy is particularly useful for studying metal complexes or organic radicals. EPR was first observed in Kazan State University by Soviet physicist Yevgeny Zavoisky in 1944, and was developed independently at the same time by Brebis Bleaney at the University of Oxford.