Non-KAM dynamical chaos in semiconductor superlattices Arkadii Krokhin, UNT
... I will present our new results concerning electron dynamics in semiconductor superlattices in the presence of non-parallel electric and magnetic field. In this geometry the electrons in the superlattice miniband turn out to form a non-KAM dynamical system that exhibits a non-traditional chaotic beha ...
... I will present our new results concerning electron dynamics in semiconductor superlattices in the presence of non-parallel electric and magnetic field. In this geometry the electrons in the superlattice miniband turn out to form a non-KAM dynamical system that exhibits a non-traditional chaotic beha ...
RADIO SPECTROSCOPY METHODS Electron spin resonance (ESR
... precession axes of the resonantly precessing spins are parallel to the axis z. If the sample is excited with a radio frequency pulse of resonance frequency, the magnetization vector of the sample will start to precess around the magnetic component of the radio frequency field. If we apply a pulse du ...
... precession axes of the resonantly precessing spins are parallel to the axis z. If the sample is excited with a radio frequency pulse of resonance frequency, the magnetization vector of the sample will start to precess around the magnetic component of the radio frequency field. If we apply a pulse du ...
Classical theory of atomic structure
... theory fails to explain the stability of atomic structure as accelerated electron radiates energy in the form of electromagnetic waves and consequently it will loose energy gradually and at last fall into the nucleus due to gravitational and coulombic attractions. Secondly classical theory fails to ...
... theory fails to explain the stability of atomic structure as accelerated electron radiates energy in the form of electromagnetic waves and consequently it will loose energy gradually and at last fall into the nucleus due to gravitational and coulombic attractions. Secondly classical theory fails to ...
Physics 105 - Multiple Choice Questions Ch 16
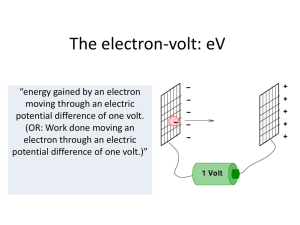
... 1. An electron volt is: A) the force acting on an electron in a field of 1 N/C B) the force required to move an electron 1 meter C) the energy gained by an electron in moving through a potential difference of 1 volt D) the energy needed to move an electron through 1 meter in any electric field E) th ...
... 1. An electron volt is: A) the force acting on an electron in a field of 1 N/C B) the force required to move an electron 1 meter C) the energy gained by an electron in moving through a potential difference of 1 volt D) the energy needed to move an electron through 1 meter in any electric field E) th ...
Atomic and Molecular Physics for Physicists Ben-Gurion University of the Negev
... Answer: µ = µB [ml + 2ms] and E=µB In the frame of the electron we are interested only in the electron spin and hence: µ = µB [2ms]= 2µB (+/- ½) Therefore: ∆E= 2µBB And B= ∆E/2µB = 4.5 10-5 / (2x 5.79 10-5) = 0.39T ...
... Answer: µ = µB [ml + 2ms] and E=µB In the frame of the electron we are interested only in the electron spin and hence: µ = µB [2ms]= 2µB (+/- ½) Therefore: ∆E= 2µBB And B= ∆E/2µB = 4.5 10-5 / (2x 5.79 10-5) = 0.39T ...
1st Semester Final Exam Review Guide
... Calculate the atomic mass of carbon based on the following mass numbers of the isotopes of carbon found in nature. mass # 12 at 81.00% mass # 13 at 10.00% mass # 14 at 9.000% 9. Define “isotope”. Give an example of 2 isotopes of a particular element. 1. In Bohr’s model of the atom, electrons that ga ...
... Calculate the atomic mass of carbon based on the following mass numbers of the isotopes of carbon found in nature. mass # 12 at 81.00% mass # 13 at 10.00% mass # 14 at 9.000% 9. Define “isotope”. Give an example of 2 isotopes of a particular element. 1. In Bohr’s model of the atom, electrons that ga ...
LEP 5.1.12 Electron spin resonance
... Now the absoption signal can be searched for: the coil current is set to about 1.3 A, button 10 “Q” pressed and the zero line again brought to the centre with 12. The signal can now be sought with 25 “C”, while continuously correcting the zero point with 12. As soon as a signal appears, both lines a ...
... Now the absoption signal can be searched for: the coil current is set to about 1.3 A, button 10 “Q” pressed and the zero line again brought to the centre with 12. The signal can now be sought with 25 “C”, while continuously correcting the zero point with 12. As soon as a signal appears, both lines a ...
Magnetic Properties of TMs So far we have seen that some
... of the d orbitals Î number of unpaired electrons. One method of determining the number of unpaired electrons is by looking at the magnetic susceptibility of a complex Î measure of the force exerted by magnetic field on a unit mass of complex is related to the population of unpaired electrons/per uni ...
... of the d orbitals Î number of unpaired electrons. One method of determining the number of unpaired electrons is by looking at the magnetic susceptibility of a complex Î measure of the force exerted by magnetic field on a unit mass of complex is related to the population of unpaired electrons/per uni ...
1.3.5 Spectroscopy Name Symbol Definition SI unit Notes total term
... The electronic states of molecules are labelled by the symmetry species label of the wavefunction in the molecular point group. These should be Latin or Greek upright capital letters. As for atoms, the spin multiplicity (2S + 1) may be indicated by a left superscript. For linear molecules the value ...
... The electronic states of molecules are labelled by the symmetry species label of the wavefunction in the molecular point group. These should be Latin or Greek upright capital letters. As for atoms, the spin multiplicity (2S + 1) may be indicated by a left superscript. For linear molecules the value ...
Nuclear Magnetic Resonance (NMR) Spectroscopy – An
... In the case of a molecule in an external magnetic field, electrons circulate and generate (small) induced magnetic fields. Electrons around a proton generate an induced field which opposes the applied field (from the spectrometer magnet) at the proton – we say the proton is ...
... In the case of a molecule in an external magnetic field, electrons circulate and generate (small) induced magnetic fields. Electrons around a proton generate an induced field which opposes the applied field (from the spectrometer magnet) at the proton – we say the proton is ...
Chapter 64: The Magnetic Moment of the Electron
... • We can interpret the particle states as sharply-peaked Gaussians, and a ...
... • We can interpret the particle states as sharply-peaked Gaussians, and a ...
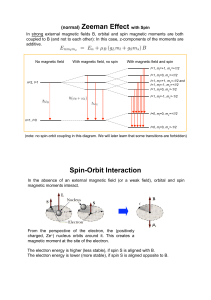
(normal) Zeeman Effect with Spin Spin
... l=1, ml=+1, ms=+1/2 l=1, ml=0, ms=+1/2 l=1, ml=+1, ms=-1/2 and l=1, ml=-1, ms=+1/2 l=1, ml=0, ms=-1/2 ...
... l=1, ml=+1, ms=+1/2 l=1, ml=0, ms=+1/2 l=1, ml=+1, ms=-1/2 and l=1, ml=-1, ms=+1/2 l=1, ml=0, ms=-1/2 ...
The Physics of Magnetic Resonance Imaging
... The external magnetic field is usually provided by large super conducting magnets operating at –269 o C and giving a field of 2T. The change in the remitted signal depends on the number of hydrogen nuclei present in the volume of the body examined. Magnetic resonance imaging (MRI) was first used in ...
... The external magnetic field is usually provided by large super conducting magnets operating at –269 o C and giving a field of 2T. The change in the remitted signal depends on the number of hydrogen nuclei present in the volume of the body examined. Magnetic resonance imaging (MRI) was first used in ...
The Physics of Magnetic Resonance Imaging
... The external magnetic field is usually provided by large super conducting magnets operating at –269 o C and giving a field of 2T. The change in the remitted signal depends on the number of hydrogen nuclei present in the volume of the body examined. Magnetic resonance imaging (MRI) was first used in ...
... The external magnetic field is usually provided by large super conducting magnets operating at –269 o C and giving a field of 2T. The change in the remitted signal depends on the number of hydrogen nuclei present in the volume of the body examined. Magnetic resonance imaging (MRI) was first used in ...
Electron paramagnetic resonance
Electron paramagnetic resonance (EPR) or electron spin resonance (ESR) spectroscopy is a technique for studying materials with unpaired electrons. The basic concepts of EPR are analogous to those of nuclear magnetic resonance (NMR), but it is electron spins that are excited instead of the spins of atomic nuclei. EPR spectroscopy is particularly useful for studying metal complexes or organic radicals. EPR was first observed in Kazan State University by Soviet physicist Yevgeny Zavoisky in 1944, and was developed independently at the same time by Brebis Bleaney at the University of Oxford.