Strain modulated microwave spectroscopy as a sensitive method to
... We have developed a new method of measuring magnetostriction constants (or components of magnetoelastic tensor) of ferromagnetic thin films. This method is based on the fact that the frequencies of ferromagnetic resonance and spin wave resonance are stress dependent. The character of this dependence ...
... We have developed a new method of measuring magnetostriction constants (or components of magnetoelastic tensor) of ferromagnetic thin films. This method is based on the fact that the frequencies of ferromagnetic resonance and spin wave resonance are stress dependent. The character of this dependence ...
Spin-Separation in Cyclotron Motion.
... initially in the higher momentum-state remains in the higher momentum-state, etc. The magnetic fields employed in the experiment (of order 0.2 T ) are small enough so that the Zeeman energy is negligible compared to the spin-orbit splitting, and only the orbital effects of the magnetic field are imp ...
... initially in the higher momentum-state remains in the higher momentum-state, etc. The magnetic fields employed in the experiment (of order 0.2 T ) are small enough so that the Zeeman energy is negligible compared to the spin-orbit splitting, and only the orbital effects of the magnetic field are imp ...
Nuclear Magnetic Resonance Spectroscopy
... 1. Wide Line Spectra Wide line spectra are those in which the band width of the source of lines is large enough so that the fine structure due to chemical environment is observed. They are useful for quantitative determination of isotopes and for studies of the physical environment of the absorbing ...
... 1. Wide Line Spectra Wide line spectra are those in which the band width of the source of lines is large enough so that the fine structure due to chemical environment is observed. They are useful for quantitative determination of isotopes and for studies of the physical environment of the absorbing ...
Homework 7
... Problem 6. A proton moves with a velocity of v = (2î − 4ĵ + k̂) m/s in a region in which the magnetic field is B = (î + 2ĵ − 3k̂) T. What is the magnitude of the magnetic force this charge experiences? ...
... Problem 6. A proton moves with a velocity of v = (2î − 4ĵ + k̂) m/s in a region in which the magnetic field is B = (î + 2ĵ − 3k̂) T. What is the magnitude of the magnetic force this charge experiences? ...
solutions
... Problem 6. A proton moves with a velocity of v = (2î − 4ĵ + k̂) m/s in a region in which the magnetic field is B = (î + 2ĵ − 3k̂) T. What is the magnitude of the magnetic force this charge experiences? ...
... Problem 6. A proton moves with a velocity of v = (2î − 4ĵ + k̂) m/s in a region in which the magnetic field is B = (î + 2ĵ − 3k̂) T. What is the magnitude of the magnetic force this charge experiences? ...
Poster
... Observations and parallel to electron spin When the direct magnetic field Bo is applied, the magnetic axis of the balll precess. Since the precession frequency is proportional to the applied field, one may want to change the voltage from the power supply to observe this effect. ...
... Observations and parallel to electron spin When the direct magnetic field Bo is applied, the magnetic axis of the balll precess. Since the precession frequency is proportional to the applied field, one may want to change the voltage from the power supply to observe this effect. ...
Effects of strain on electron spin transport in semiconductor epilayers
... Theory of Condensed Matter Group, Cavendish Laboratory, Cambridge, CB3 0HE, United Kingdom Los Alamos National Laboratory, Los Alamos, New Mexico 87544, USA ...
... Theory of Condensed Matter Group, Cavendish Laboratory, Cambridge, CB3 0HE, United Kingdom Los Alamos National Laboratory, Los Alamos, New Mexico 87544, USA ...
L-J Chemistry 1 Quiz 25 1 A property that depends on the amount of
... Device to break light down into a spectrum Electrons in the outer level of an atom that can be gained, shared or lost Energy added to an atom when removing an electron Group of atoms held together by covalent bonds A compound composed of four different atoms A polyatomic ion containing oxygen Formul ...
... Device to break light down into a spectrum Electrons in the outer level of an atom that can be gained, shared or lost Energy added to an atom when removing an electron Group of atoms held together by covalent bonds A compound composed of four different atoms A polyatomic ion containing oxygen Formul ...
THE SOCIETY FOR ANALYTICAL CHEMISTRY PHYSICAL
... compounds. Measurements are outlined here which show that the technique can now be extended to cover many different types of radicals formed by breakage of bonds with U.V. irradiation. This method can be applied quite generally to any system in which the resultant radicals can be trapped and observe ...
... compounds. Measurements are outlined here which show that the technique can now be extended to cover many different types of radicals formed by breakage of bonds with U.V. irradiation. This method can be applied quite generally to any system in which the resultant radicals can be trapped and observe ...
QUIZ 4 ... Formulas and constants Mass of electron = 9.1. 10
... Professor S.K.Sinha Formulas and constants Mass of electron = 9.1. 10 -31 kg Charge on electron = 1.6.10-19 C Planck’s Constant h= 6.626. 10-34 J.s =4.136. 10-15 eV.s h / 2 1.055.10 34 J.s 6.582.10 16 eV.s ...
... Professor S.K.Sinha Formulas and constants Mass of electron = 9.1. 10 -31 kg Charge on electron = 1.6.10-19 C Planck’s Constant h= 6.626. 10-34 J.s =4.136. 10-15 eV.s h / 2 1.055.10 34 J.s 6.582.10 16 eV.s ...
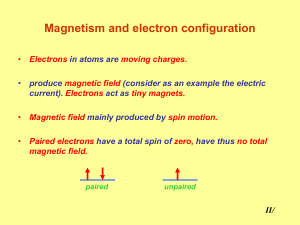
Magnetism and electron configuration
... Magnetism and electron configuration • Electrons in atoms are moving charges. • produce magnetic field (consider as an example the electric current). Electrons act as tiny magnets. ...
... Magnetism and electron configuration • Electrons in atoms are moving charges. • produce magnetic field (consider as an example the electric current). Electrons act as tiny magnets. ...
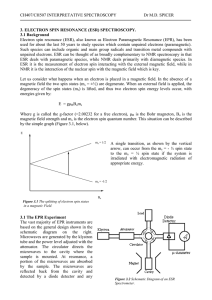
DETECTION OF UNPAIRED ELECTRONS
... bonding information about molecules in the gas phase.) Depending on the environment of the unpaired electron, it may be more susceptible or less susceptible to the influence of the external magnetic field. That means the energy splitting between the two spin states will vary from one molecule to ano ...
... bonding information about molecules in the gas phase.) Depending on the environment of the unpaired electron, it may be more susceptible or less susceptible to the influence of the external magnetic field. That means the energy splitting between the two spin states will vary from one molecule to ano ...
Formulas and constants Mass of electron m = 9.1. 10 kg
... Formulas and constants Mass of electron me = 9.1. 10 -31 kg Charge on electron = 1.6.10-19 C Planck’s Constant h= 6.626. 10-34 J.s =4.136. 10-15 eV.s h = h / 2! = 1.055.10 "34 J.s = 6.582.10 "16 eV.s ...
... Formulas and constants Mass of electron me = 9.1. 10 -31 kg Charge on electron = 1.6.10-19 C Planck’s Constant h= 6.626. 10-34 J.s =4.136. 10-15 eV.s h = h / 2! = 1.055.10 "34 J.s = 6.582.10 "16 eV.s ...
PhD position: Dynamic Nuclear Polarization using Electron-Nuclear Double Resonance
... noise. However, it is necessary to increase the averaging time by a factor of N to obtain merely a √N increase in the signal-to-noise ratio. This means that if we can increase the signal by a factor of 1,000 it would correspond to a huge speed up of the NMR experiment by a factor of 1,000,000. DNP i ...
... noise. However, it is necessary to increase the averaging time by a factor of N to obtain merely a √N increase in the signal-to-noise ratio. This means that if we can increase the signal by a factor of 1,000 it would correspond to a huge speed up of the NMR experiment by a factor of 1,000,000. DNP i ...
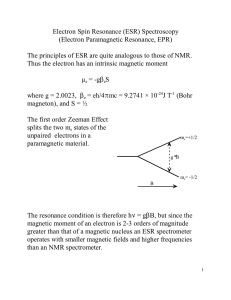
Electron paramagnetic resonance
Electron paramagnetic resonance (EPR) or electron spin resonance (ESR) spectroscopy is a technique for studying materials with unpaired electrons. The basic concepts of EPR are analogous to those of nuclear magnetic resonance (NMR), but it is electron spins that are excited instead of the spins of atomic nuclei. EPR spectroscopy is particularly useful for studying metal complexes or organic radicals. EPR was first observed in Kazan State University by Soviet physicist Yevgeny Zavoisky in 1944, and was developed independently at the same time by Brebis Bleaney at the University of Oxford.