Periodic Properties of the Elements
... Description of Activity In this activity, a partial periodic table is provided. You will select an element and evaluate the element’s physical properties. After selecting the element, you will analyze the patterns of these properties as they occur across a period as well as trends that exist down a ...
... Description of Activity In this activity, a partial periodic table is provided. You will select an element and evaluate the element’s physical properties. After selecting the element, you will analyze the patterns of these properties as they occur across a period as well as trends that exist down a ...
Inorganic Chemistry ELEMENTS AND
... Anion :It is a negatively charged ion which is formed when an atom gains one or more electrons, e.g. F- , O2- , S2- etc. Chemical Bond : Chemical bond may be defined as the attractive force that binds together the constituent atoms in a molecule. Following are some different types of chemical bonds ...
... Anion :It is a negatively charged ion which is formed when an atom gains one or more electrons, e.g. F- , O2- , S2- etc. Chemical Bond : Chemical bond may be defined as the attractive force that binds together the constituent atoms in a molecule. Following are some different types of chemical bonds ...
Chemistry Online Textbook
... an atom's mass is held. Although the nucleus contains the majority of the mass of the atom, the nucleus is very small compared to the size of the whole atom, because most of the atom is empty space surrounding the nucleus. The nucleus is made up of two smaller particles called protons and neutrons. ...
... an atom's mass is held. Although the nucleus contains the majority of the mass of the atom, the nucleus is very small compared to the size of the whole atom, because most of the atom is empty space surrounding the nucleus. The nucleus is made up of two smaller particles called protons and neutrons. ...
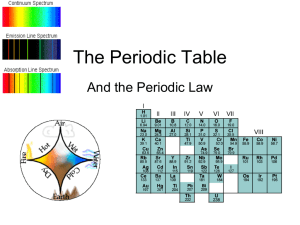
The Periodic Table
... – All elements have something in common if they are in the same row. All of the elements in a period have the same number of atomic orbitals or electron shells. Every element in the top row (the first period) has one orbital or shell for its electrons. All of the elements in the second row (the seco ...
... – All elements have something in common if they are in the same row. All of the elements in a period have the same number of atomic orbitals or electron shells. Every element in the top row (the first period) has one orbital or shell for its electrons. All of the elements in the second row (the seco ...
File
... -------------1. The ion is positively charged and its radius is smaller than the radius of the atom. 2. The ion is positively charged and its radius 5. is larger than the radius of the atom. Which two characteristics are associated with 3. The ion is negatively charged and its radius metals? is smal ...
... -------------1. The ion is positively charged and its radius is smaller than the radius of the atom. 2. The ion is positively charged and its radius 5. is larger than the radius of the atom. Which two characteristics are associated with 3. The ion is negatively charged and its radius metals? is smal ...
11. Patterns in the Periodic Table
... Where were the elements made? There are 92 naturally-occurring elements and about 15 artificially-produced elements. Elements were originally made in stars. In the early stages of a star’s life, light elements, such as hydrogen and helium, are formed. These fused together to make heavier elements s ...
... Where were the elements made? There are 92 naturally-occurring elements and about 15 artificially-produced elements. Elements were originally made in stars. In the early stages of a star’s life, light elements, such as hydrogen and helium, are formed. These fused together to make heavier elements s ...
File - eScience@Kings
... Where were the elements made? There are 92 naturally-occurring elements and about 15 artificially-produced elements. Elements were originally made in stars. In the early stages of a star’s life, light elements, such as hydrogen and helium, are formed. These fused together to make heavier elements s ...
... Where were the elements made? There are 92 naturally-occurring elements and about 15 artificially-produced elements. Elements were originally made in stars. In the early stages of a star’s life, light elements, such as hydrogen and helium, are formed. These fused together to make heavier elements s ...
11. Patterns in the Periodic Table
... Where were the elements made? There are 92 naturally-occurring elements and about 15 artificially-produced elements. Elements were originally made in stars. In the early stages of a star’s life, light elements, such as hydrogen and helium, are formed. These fused together to make heavier elements s ...
... Where were the elements made? There are 92 naturally-occurring elements and about 15 artificially-produced elements. Elements were originally made in stars. In the early stages of a star’s life, light elements, such as hydrogen and helium, are formed. These fused together to make heavier elements s ...
Introductory Chemistry: Concepts & Connections 4th Edition
... Introductory Chemistry: Concepts & Connections 4th Edition by Charles H. Corwin ...
... Introductory Chemistry: Concepts & Connections 4th Edition by Charles H. Corwin ...
Periodic Table
... Introductory Chemistry: Concepts & Connections 4th Edition by Charles H. Corwin ...
... Introductory Chemistry: Concepts & Connections 4th Edition by Charles H. Corwin ...
Periodic Table Notes
... grouped into families based on their chemical properties. Each family has a specific name to differentiate it from the other families in the periodic table. Elements are classified as metals, nonmetals, and metalloids, by their properties. Metals are found to the left of the zigzag line. Atoms of mo ...
... grouped into families based on their chemical properties. Each family has a specific name to differentiate it from the other families in the periodic table. Elements are classified as metals, nonmetals, and metalloids, by their properties. Metals are found to the left of the zigzag line. Atoms of mo ...
File - CCHS Chemistry
... In the periodic table, elems w/ similar props are in a column An atom’s chemical props are determined by its e- config ...
... In the periodic table, elems w/ similar props are in a column An atom’s chemical props are determined by its e- config ...
UNIT-3 Classification of elements and periodicity
... 9. How many elements are there in the 4th period of long form of periodic table? 10. Write the atomic number of the element unniltrium. 11. Give the IUPAC name of the element whose atomic number is 109? 12. Which one of the following subshell is not filled in the 5th period (5s, 5p, 5d, 4d)? 13. In ...
... 9. How many elements are there in the 4th period of long form of periodic table? 10. Write the atomic number of the element unniltrium. 11. Give the IUPAC name of the element whose atomic number is 109? 12. Which one of the following subshell is not filled in the 5th period (5s, 5p, 5d, 4d)? 13. In ...
Chapter 6 the Periodic Table
... • Metals- good conductor of heat and electricity, shiny luster, solid at room temperature (except Hg), Malleable and ductile, high boiling and melting points, High densities. • Non-metals- Generally gasses at room temperature , Poor conductors of heat and electricity, Brittle dull-looking when sol ...
... • Metals- good conductor of heat and electricity, shiny luster, solid at room temperature (except Hg), Malleable and ductile, high boiling and melting points, High densities. • Non-metals- Generally gasses at room temperature , Poor conductors of heat and electricity, Brittle dull-looking when sol ...
Placing Elements on the Periodic Table
... grouped into families based on their chemical properties. Each family has a specific name to differentiate it from the other families in the periodic table. Elements are classified as metals, nonmetals, and metalloids, by their properties. Metals are found to the left of the zigzag line. Atoms of mo ...
... grouped into families based on their chemical properties. Each family has a specific name to differentiate it from the other families in the periodic table. Elements are classified as metals, nonmetals, and metalloids, by their properties. Metals are found to the left of the zigzag line. Atoms of mo ...
Introduction to Atoms
... 3. Is the following sentence true or false? Several of the nonmetals are gases at room temperature. ________________________ 4. Circle the letter of each sentence that is true about the physical properties of nonmetals. a. Solid nonmetals are brittle. b. They usually have lower densities than metals ...
... 3. Is the following sentence true or false? Several of the nonmetals are gases at room temperature. ________________________ 4. Circle the letter of each sentence that is true about the physical properties of nonmetals. a. Solid nonmetals are brittle. b. They usually have lower densities than metals ...
Review Topic 3: Elements, Radioactivity, and the Periodic Table
... A 3. Which of these describes a tendency for atomic radii as displayed on the periodic chart? a. Atomic radii decrease left to right across a period. b. Atomic radii increase left to right across a period. c. Atomic radii decrease top to bottom down a group. d. Atomic radii increase, then decrea ...
... A 3. Which of these describes a tendency for atomic radii as displayed on the periodic chart? a. Atomic radii decrease left to right across a period. b. Atomic radii increase left to right across a period. c. Atomic radii decrease top to bottom down a group. d. Atomic radii increase, then decrea ...
Addrienne`s Element Lesson Plan
... Definition: A solid substance made by mixing a metal with another substance, usually another metal, to have specific properties that metals alone lack Context: The earliest metalworkers combined different elemental metals in search of the best alloys for weapons and tools. element Definition: A subs ...
... Definition: A solid substance made by mixing a metal with another substance, usually another metal, to have specific properties that metals alone lack Context: The earliest metalworkers combined different elemental metals in search of the best alloys for weapons and tools. element Definition: A subs ...
Periods and Blocks of the Periodic Table
... • In many compounds, the negative charge of the valence electrons is concentrated closer to one atom than to another. • Electronegativity is a measure of the ability of an atom in a chemical compound to attract electrons from another atom in the compound. • Electronegativities tend to increase acros ...
... • In many compounds, the negative charge of the valence electrons is concentrated closer to one atom than to another. • Electronegativity is a measure of the ability of an atom in a chemical compound to attract electrons from another atom in the compound. • Electronegativities tend to increase acros ...
Example 1-2
... 1. All Group IA salts are soluble (aq). 2. All ammonium salts are soluble (aq). 3. All salts containing nitrate, acetate, chlorate and perchlorate are soluble (aq). 4. All salts containing halides (chlorides, bromides, iodides, and fluorides) are soluble (aq) EXCEPT silver, mercury(I) and lead (s). ...
... 1. All Group IA salts are soluble (aq). 2. All ammonium salts are soluble (aq). 3. All salts containing nitrate, acetate, chlorate and perchlorate are soluble (aq). 4. All salts containing halides (chlorides, bromides, iodides, and fluorides) are soluble (aq) EXCEPT silver, mercury(I) and lead (s). ...
2. CHEMICAL ACTIVITY of the METALS 3. PATTERNS of the
... The ionic compounds formed are collectively known as “salts”, so the general pattern of the reactions is ...
... The ionic compounds formed are collectively known as “salts”, so the general pattern of the reactions is ...
Work Booklet - Brooks Composite High School
... 4. Study the display your teacher has provided, showing products that many people use in their homes. Look for symbols that are HHPS. Read the label on each product to find out how the product is used and decide why it has an HHPS. Complete the table below. ...
... 4. Study the display your teacher has provided, showing products that many people use in their homes. Look for symbols that are HHPS. Read the label on each product to find out how the product is used and decide why it has an HHPS. Complete the table below. ...
Electrons in Atoms - Effingham County Schools
... B. In a third-period element, the highest occupied energy level is the third main energy level, n = 3. The 1s, 2s, and 2p sublevels are completely filled ...
... B. In a third-period element, the highest occupied energy level is the third main energy level, n = 3. The 1s, 2s, and 2p sublevels are completely filled ...