File
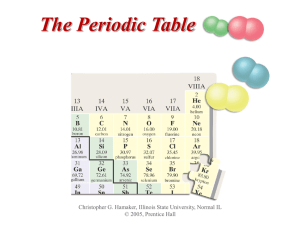
... The seven or eight horizontal rows of the Periodic Table are called periods. The first period is the shortest and has only two elements, hydrogen and helium. The sixth horizontal row or period contains 32 elements. The left most element in a period, or row, has just one electron in its outer shell, ...
... The seven or eight horizontal rows of the Periodic Table are called periods. The first period is the shortest and has only two elements, hydrogen and helium. The sixth horizontal row or period contains 32 elements. The left most element in a period, or row, has just one electron in its outer shell, ...
Document
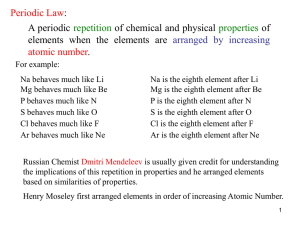
... • The elements were first organized by increasing atomic mass, which led to inconsistencies. Later, they were organized by increasing atomic number. • The periodic law states that when the elements are arranged by increasing atomic number, there is a periodic repetition of their chemical and physica ...
... • The elements were first organized by increasing atomic mass, which led to inconsistencies. Later, they were organized by increasing atomic number. • The periodic law states that when the elements are arranged by increasing atomic number, there is a periodic repetition of their chemical and physica ...
The Periodic Table - Anderson High School
... Groups…Here’s Where the Periodic Table Gets Useful!! • Elements in the same group have similar chemical and physical properties!! (Mendeleev did that on purpose.) • Elements in the”A” groups are called representative elements. ...
... Groups…Here’s Where the Periodic Table Gets Useful!! • Elements in the same group have similar chemical and physical properties!! (Mendeleev did that on purpose.) • Elements in the”A” groups are called representative elements. ...
The Periodic Table
... Groups…Here’s Where the Periodic Table Gets Useful!! • Elements in the same group have similar chemical and physical properties!! (Mendeleev did that on purpose.) • Elements in the”A” groups are called representative elements. ...
... Groups…Here’s Where the Periodic Table Gets Useful!! • Elements in the same group have similar chemical and physical properties!! (Mendeleev did that on purpose.) • Elements in the”A” groups are called representative elements. ...
The Periodic Table
... The Noble Gases • The periodic table was expanded by one group at the far right of the periodic table with the discovery of argon in 1894. • Helium, neon, krypton, xenon, and radon were subsequently discovered in the next 5 years. • They were originally called the inert gases. • Recently, several c ...
... The Noble Gases • The periodic table was expanded by one group at the far right of the periodic table with the discovery of argon in 1894. • Helium, neon, krypton, xenon, and radon were subsequently discovered in the next 5 years. • They were originally called the inert gases. • Recently, several c ...
Periodic Table Oakland Schools Chemistry Resource Unit Andrew D. Hulbert
... Periodic Table - in the periodic table, elements are arranged in order of increasing number of protons (called the atomic number). Vertical groups in the periodic table (families) have similar physical and chemical properties due to the same outer electron ...
... Periodic Table - in the periodic table, elements are arranged in order of increasing number of protons (called the atomic number). Vertical groups in the periodic table (families) have similar physical and chemical properties due to the same outer electron ...
Unit Expectations – Periodic Table
... Law States: In the periodic table, elements are arranged in order of increasing number of protons (called the atomic number). Vertical groups in the periodic table (families) have similar physical and chemical properties due to the same outer electron structures, 9. C4.9A Introduced: _______ Basic: ...
... Law States: In the periodic table, elements are arranged in order of increasing number of protons (called the atomic number). Vertical groups in the periodic table (families) have similar physical and chemical properties due to the same outer electron structures, 9. C4.9A Introduced: _______ Basic: ...
Study Material - Tiwariacademy.net
... When elements are arranged it was found that every eighth element had properties similar to that of the first. eg properties of sodium and Lithium are the same. ...
... When elements are arranged it was found that every eighth element had properties similar to that of the first. eg properties of sodium and Lithium are the same. ...
Group 16: The Oxygen Family - Chemwiki
... with up to 6 atoms. In the presence of hydrogen it forms the compound hydrogen sulfide, H2S, a poisonous gas incapable of forming hydrogen bonds and with a very small dipole moment. Hydrogen sulfide can easily be recognized by its strong odor that is similar to that of rotten eggs, but this smell ca ...
... with up to 6 atoms. In the presence of hydrogen it forms the compound hydrogen sulfide, H2S, a poisonous gas incapable of forming hydrogen bonds and with a very small dipole moment. Hydrogen sulfide can easily be recognized by its strong odor that is similar to that of rotten eggs, but this smell ca ...
Science 2nd prep. 1st term Lesson 2 Graduation of the properties of
... The ability of the oxygen atom to attract e-covalent bonds (OH) is greater than the capacity of the hydrogen atom because the electronegativity of oxygen (3.5) is greater than Alsalbeh hydrogen (2.1) Water from the polar covalent compounds, because difference electronegativity ...
... The ability of the oxygen atom to attract e-covalent bonds (OH) is greater than the capacity of the hydrogen atom because the electronegativity of oxygen (3.5) is greater than Alsalbeh hydrogen (2.1) Water from the polar covalent compounds, because difference electronegativity ...
Periodic Trends Notes 14-15
... • Describe the arrangement of elements in the periodic table in order of increasing atomic number. • Distinguish between the terms group and period. • Apply the relationship between the electron arrangement of elements and their position in the periodic table. • Apply the relationship between the nu ...
... • Describe the arrangement of elements in the periodic table in order of increasing atomic number. • Distinguish between the terms group and period. • Apply the relationship between the electron arrangement of elements and their position in the periodic table. • Apply the relationship between the nu ...
Periodic Table Notes.notebook
... charge of the nucleus from outer electrons resulting in those outer electrons not being as tightly bound to the atom. Ionization Energy Ionization energy is the amount of energy required to remove the outmost electron. It is closely related to electronegativity. Period ionization energy incre ...
... charge of the nucleus from outer electrons resulting in those outer electrons not being as tightly bound to the atom. Ionization Energy Ionization energy is the amount of energy required to remove the outmost electron. It is closely related to electronegativity. Period ionization energy incre ...
Slide 1
... How did chemists begin to organize the known elements? How did Mendeleev organize his periodic table? How is the modern periodic table organized? What are three broad classes of elements? ...
... How did chemists begin to organize the known elements? How did Mendeleev organize his periodic table? How is the modern periodic table organized? What are three broad classes of elements? ...
Periodic Table
... 1. In the past people believed that there were only 4 elements now we know that there are over 100 elements 2. Everything is made of elements 3. A reason of your own. ...
... 1. In the past people believed that there were only 4 elements now we know that there are over 100 elements 2. Everything is made of elements 3. A reason of your own. ...
Increasing Radii
... Group nonmetals, all have s2, p6 electron configuration (except He) Nonreactive elements-almost no know compounds containing these elements. Remember that a full energy level is not reactive. ...
... Group nonmetals, all have s2, p6 electron configuration (except He) Nonreactive elements-almost no know compounds containing these elements. Remember that a full energy level is not reactive. ...
GCSE - WordPress.com
... 4. Explain the following in terms of bonding and structure ideas :. (i) Silicon dioxide and carbon dioxide both contain covalent bonds but the former melts at 1700oC whereas the latter is a gas at 0oC. (ii) Sodium oxide, carbon dioxide and silicon dioxide are all poor conductors of electricity ...
... 4. Explain the following in terms of bonding and structure ideas :. (i) Silicon dioxide and carbon dioxide both contain covalent bonds but the former melts at 1700oC whereas the latter is a gas at 0oC. (ii) Sodium oxide, carbon dioxide and silicon dioxide are all poor conductors of electricity ...
Homework Booklet [4,S]
... 4. Explain the following in terms of bonding and structure ideas :. (i) Silicon dioxide and carbon dioxide both contain covalent bonds but the former melts at 1700oC whereas the latter is a gas at 0oC. (ii) Sodium oxide, carbon dioxide and silicon dioxide are all poor conductors of electricity ...
... 4. Explain the following in terms of bonding and structure ideas :. (i) Silicon dioxide and carbon dioxide both contain covalent bonds but the former melts at 1700oC whereas the latter is a gas at 0oC. (ii) Sodium oxide, carbon dioxide and silicon dioxide are all poor conductors of electricity ...
Chapter 7 Periodic Properties of Elements - GCG-42
... solids and reactive non-metals. Each period ends with a non-reactive noble gas ...
... solids and reactive non-metals. Each period ends with a non-reactive noble gas ...
MENDELEEV AND THE ATOMIC TABLE Dmitri Ivanovich
... oxygen and selenium are in the same column because they both have 4 electrons in the outermost shell. In general, elements with similar chemical properties fall into the same group in the periodic table, Thus it is relatively easy to predict the chemical properties of an element if one knows its pos ...
... oxygen and selenium are in the same column because they both have 4 electrons in the outermost shell. In general, elements with similar chemical properties fall into the same group in the periodic table, Thus it is relatively easy to predict the chemical properties of an element if one knows its pos ...
Oct 17-Oct 21
... I can label a periodic table with oxidation numbers of main group elements, identify elements likely to form ions and use information to construct formulas for compounds ...
... I can label a periodic table with oxidation numbers of main group elements, identify elements likely to form ions and use information to construct formulas for compounds ...
Periodic Table Study Guide
... 15) What are valence electrons? Why are they important? 16) What is the pattern of valence electrons in the periodic table? What groups is this true for? ...
... 15) What are valence electrons? Why are they important? 16) What is the pattern of valence electrons in the periodic table? What groups is this true for? ...
Learning Guide 3
... energy, and metallic and nonmetallic properties. • Each period shows trends in atomic radius, Electronegativity, first ionization energy, and metallic and nonmetallic properties. • When an element becomes an anion by gaining electrons, the radius increases. • When an element becomes a cation by losi ...
... energy, and metallic and nonmetallic properties. • Each period shows trends in atomic radius, Electronegativity, first ionization energy, and metallic and nonmetallic properties. • When an element becomes an anion by gaining electrons, the radius increases. • When an element becomes a cation by losi ...
Patterns in The Periodic Table
... Because atoms are neutral in charge, the number of negatively charged electrons must equal the number of positively ...
... Because atoms are neutral in charge, the number of negatively charged electrons must equal the number of positively ...













![Homework Booklet [4,S]](http://s1.studyres.com/store/data/010355871_1-63c750e3d1b58eaaebbb3f5d45651c44-300x300.png)