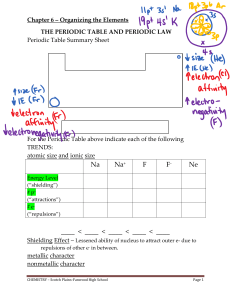
The Hydroxylation of Aromatic Nitro Compounds by Alkalies
... When 16 g of I-nitronaphthalene was stirred mechanically with 26 g of potassium hydroxide and 50 ml of benzene tor tlve hours at 70°, 1 g of 1·nitro-2-naphthol. m.p. 103°. was obtained. Ten g of !-nitrobiphenJ/l was stirred with 100 g of potassium hydroxide and 100 ml of benzene for five hours at 80 ...
... When 16 g of I-nitronaphthalene was stirred mechanically with 26 g of potassium hydroxide and 50 ml of benzene tor tlve hours at 70°, 1 g of 1·nitro-2-naphthol. m.p. 103°. was obtained. Ten g of !-nitrobiphenJ/l was stirred with 100 g of potassium hydroxide and 100 ml of benzene for five hours at 80 ...
Chapter 5: Electrons
... The metalloids divide the metals from the nonmetals. They are mostly brittle solids with some properties of metals and some of nonmetals. The electrical conductivity falls between the metals and nonmetals. The metals of the p block are reactive enough to be found in nature only as compounds and not ...
... The metalloids divide the metals from the nonmetals. They are mostly brittle solids with some properties of metals and some of nonmetals. The electrical conductivity falls between the metals and nonmetals. The metals of the p block are reactive enough to be found in nature only as compounds and not ...
chapter 5-Chemical Periodicity
... covalent binary compounds. • One example is the haloacids鹵化酸類produced by the reaction of hydrogen with the halogens 鹵素. H2 + X2 2 HX • For example, the reactions of F2 and Br2 with H2 are: ...
... covalent binary compounds. • One example is the haloacids鹵化酸類produced by the reaction of hydrogen with the halogens 鹵素. H2 + X2 2 HX • For example, the reactions of F2 and Br2 with H2 are: ...
Periodic Trends PDF - Warren County Schools
... • This group of metals is extremely reactive! This is due to the fact that they all have only 1 valence electron. • In their pure state, all members of this group are silvery in appearance and soft enough to be cut with a butter knife. • Because they are so reactive, they are not found in natur ...
... • This group of metals is extremely reactive! This is due to the fact that they all have only 1 valence electron. • In their pure state, all members of this group are silvery in appearance and soft enough to be cut with a butter knife. • Because they are so reactive, they are not found in natur ...
The Periodic Table - Warren County Public Schools
... • This group of metals is extremely reactive! This is due to the fact that they all have only 1 valence electron. • In their pure state, all members of this group are silvery in appearance and soft enough to be cut with a butter knife. • Because they are so reactive, they are not found in nature as ...
... • This group of metals is extremely reactive! This is due to the fact that they all have only 1 valence electron. • In their pure state, all members of this group are silvery in appearance and soft enough to be cut with a butter knife. • Because they are so reactive, they are not found in nature as ...
Unit 1 Summary - A
... Chemical equations (e) construct balanced chemical equations for reactions studied and for unfamiliar ...
... Chemical equations (e) construct balanced chemical equations for reactions studied and for unfamiliar ...
C1a 1.1 Atoms, Elements and Compounds
... between atomic mass and the density of an element. He plotted a graph of atomic volume against relative atomic mass and obtained a curve with peaks and troughs. Elements with similar properties were in similar places on the ...
... between atomic mass and the density of an element. He plotted a graph of atomic volume against relative atomic mass and obtained a curve with peaks and troughs. Elements with similar properties were in similar places on the ...
The Periodic Table and Periodic Law
... What does a group tell us about valence electrons and ion formation? ...
... What does a group tell us about valence electrons and ion formation? ...
Summer Study Assignment – Honors Chem 2/AP Chemistry
... 31. Write the electron configuration using the Noble Gas core method for californium. 32. Write a balanced equation for the following double replacement reactions: a. Calcium hydroxide (aq) + nitric acid (aq) b. Chromium (III) sulfite (aq) + sulfuric acid (aq) c. Zinc chloride (aq) + ammonium su ...
... 31. Write the electron configuration using the Noble Gas core method for californium. 32. Write a balanced equation for the following double replacement reactions: a. Calcium hydroxide (aq) + nitric acid (aq) b. Chromium (III) sulfite (aq) + sulfuric acid (aq) c. Zinc chloride (aq) + ammonium su ...
Periodic Trends Studyguide with Questions and Answers
... MY2 is the general formula of a Group 2 atom bonding with a Group 17 halogen (Y) MO is the general formula of a Group 2 atom bonding with O (to form an oxide) . Radium (Ra) is the most reactive metal in this group. Radium is also radioactive. . All alkaline earth metals exist as solids at STP ...
... MY2 is the general formula of a Group 2 atom bonding with a Group 17 halogen (Y) MO is the general formula of a Group 2 atom bonding with O (to form an oxide) . Radium (Ra) is the most reactive metal in this group. Radium is also radioactive. . All alkaline earth metals exist as solids at STP ...
Periodic Table
... • The actual amount of EN an atom has is indicated by a number on the Pauling Electronegativity Scale that goes from 0 to 4. • Dr. Linus Pauling set up this scale and gave the element having the greatest EN an arbitrary number of 4, and he assigned numbers to the others relative to this element. ...
... • The actual amount of EN an atom has is indicated by a number on the Pauling Electronegativity Scale that goes from 0 to 4. • Dr. Linus Pauling set up this scale and gave the element having the greatest EN an arbitrary number of 4, and he assigned numbers to the others relative to this element. ...
Mendeleev`s Periodic Table - Scotch Plains
... Have dispersion forces between molecules (weak forces of attraction). Dispersion forces increase with larger mass, so boiling point of Kr is higher than He Ions: ions do not have equal number of protons and electrons as atoms do + ions= less electrons than protons (+1 charge indicates one lost elect ...
... Have dispersion forces between molecules (weak forces of attraction). Dispersion forces increase with larger mass, so boiling point of Kr is higher than He Ions: ions do not have equal number of protons and electrons as atoms do + ions= less electrons than protons (+1 charge indicates one lost elect ...
Periodic Table
... • The actual amount of EN an atom has is indicated by a number on the Pauling Electronegativity Scale that goes from 0 to 4. • Dr. Linus Pauling set up this scale and gave the element having the greatest EN an arbitrary number of 4, and he assigned numbers to the others relative to this element. ...
... • The actual amount of EN an atom has is indicated by a number on the Pauling Electronegativity Scale that goes from 0 to 4. • Dr. Linus Pauling set up this scale and gave the element having the greatest EN an arbitrary number of 4, and he assigned numbers to the others relative to this element. ...
서울대학교 일반화학실험
... was reported as 223.3. Let's consider the significance of Ra/Cl = 3.154. The weight of Cl in 0.10925 g RaCl2 must have been derived from the weight of Cl in AgCl. The difference from 0.10925 g should be the weight of Ra. weight of Ra in 0.10925 g RaCl2 : 0.10925 x (3.154)/(4.154) = ...
... was reported as 223.3. Let's consider the significance of Ra/Cl = 3.154. The weight of Cl in 0.10925 g RaCl2 must have been derived from the weight of Cl in AgCl. The difference from 0.10925 g should be the weight of Ra. weight of Ra in 0.10925 g RaCl2 : 0.10925 x (3.154)/(4.154) = ...
handout 4
... A sample of CH4(g) having a volume of 2.80 Liters at 25°C and 1.65 atm, was ignited with a sample of oxygen gas having a volume of 35.0 Liters at 31°C and 1.25 atm to produce CO2 and H2O vapor. Calculate the volume of CO2 formed at a pressure of 2.50 atm and a temperature of 125°C. ...
... A sample of CH4(g) having a volume of 2.80 Liters at 25°C and 1.65 atm, was ignited with a sample of oxygen gas having a volume of 35.0 Liters at 31°C and 1.25 atm to produce CO2 and H2O vapor. Calculate the volume of CO2 formed at a pressure of 2.50 atm and a temperature of 125°C. ...
The physical characteristics of the atom of an element are called
... atomic masses. The properties of every eight elements were similar to the first one, like the eighth note of a musical scale.This repetition in the properties of elements is just like the repetition of eighth node in an octave of music. 3. Mendeleev's Periodic Law:The physical and chemical propertie ...
... atomic masses. The properties of every eight elements were similar to the first one, like the eighth note of a musical scale.This repetition in the properties of elements is just like the repetition of eighth node in an octave of music. 3. Mendeleev's Periodic Law:The physical and chemical propertie ...
The Periodic Table and Periodic Law
... decreases down a group because atomic size increases and less energy is required to remove an electron farther from the nucleus. ...
... decreases down a group because atomic size increases and less energy is required to remove an electron farther from the nucleus. ...
The Periodic Table and Periodic Law
... of identical atoms that are chemically bonded together. – Atomic Size/Radius decreases as you move left to right across the period… this is because there is an increased nuclear charge (more protons pulling the outer electrons closer to the nucleus). – Atomic Size/Radius increases as you move top to ...
... of identical atoms that are chemically bonded together. – Atomic Size/Radius decreases as you move left to right across the period… this is because there is an increased nuclear charge (more protons pulling the outer electrons closer to the nucleus). – Atomic Size/Radius increases as you move top to ...
Chapter 7 Outline full
... Metals are shiny and lustrous, malleable and ductile. Metals are solids at room temperature (exception: mercury is liquid at room temperature; gallium and cesium melt just above room temperature) and have very high melting temperatures. Metals tend to have low ionization energies and tend to form ca ...
... Metals are shiny and lustrous, malleable and ductile. Metals are solids at room temperature (exception: mercury is liquid at room temperature; gallium and cesium melt just above room temperature) and have very high melting temperatures. Metals tend to have low ionization energies and tend to form ca ...
Chapter 7 Outline full
... Metals are shiny and lustrous, malleable and ductile. Metals are solids at room temperature (exception: mercury is liquid at room temperature; gallium and cesium melt just above room temperature) and have very high melting temperatures. Metals tend to have low ionization energies and tend to form ca ...
... Metals are shiny and lustrous, malleable and ductile. Metals are solids at room temperature (exception: mercury is liquid at room temperature; gallium and cesium melt just above room temperature) and have very high melting temperatures. Metals tend to have low ionization energies and tend to form ca ...
Chemical Periodicity
... 2. Who is Dmitri Mendeleev, and what was his contribution to chemistry? 3. How is the modern periodic table different from the first periodic table? 4. What is the modern periodic law? 5. Organization of the periodic table. Indicate where the following are located on the periodic table. h. Transitio ...
... 2. Who is Dmitri Mendeleev, and what was his contribution to chemistry? 3. How is the modern periodic table different from the first periodic table? 4. What is the modern periodic law? 5. Organization of the periodic table. Indicate where the following are located on the periodic table. h. Transitio ...