OXIDATION AND REDUCTION
... Oxidation Number is the valency of an atom in a molecule or ion which is assigned the sign either + or -. It may be (i)electrovalency or (ii)covalency . When the proper sign is associated with valency it becomes oxidation number(ON). It is mostly a theoretical concept particularly for covalent compo ...
... Oxidation Number is the valency of an atom in a molecule or ion which is assigned the sign either + or -. It may be (i)electrovalency or (ii)covalency . When the proper sign is associated with valency it becomes oxidation number(ON). It is mostly a theoretical concept particularly for covalent compo ...
The d- and f- Block Element Block Elements The d- and f
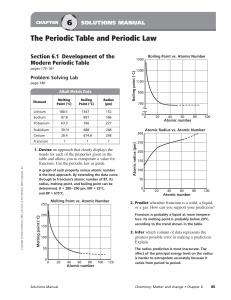
... in Fig. 8.3 show an increase from the first (3d) to the second (4d) series of the elements but the radii of the third (5d) series are virtually the same as those of the corresponding members of the second series. This phenomenon is associated with the intervention of the 4f orbitals which must be fi ...
... in Fig. 8.3 show an increase from the first (3d) to the second (4d) series of the elements but the radii of the third (5d) series are virtually the same as those of the corresponding members of the second series. This phenomenon is associated with the intervention of the 4f orbitals which must be fi ...
Chapter 2 - UBC Physics
... alkali metals first (combining power of one), alkaline earths (two), etc. However, it was difficult to classify metals such as copper and mercury which had multiple combining powers, sometimes one and other times two. While trying to sort out this dilemma, Mendeleev noticed patterns in the propertie ...
... alkali metals first (combining power of one), alkaline earths (two), etc. However, it was difficult to classify metals such as copper and mercury which had multiple combining powers, sometimes one and other times two. While trying to sort out this dilemma, Mendeleev noticed patterns in the propertie ...
Chemistry A- Periodic Table Packet
... and good conductors of heat and electricity. They also have higher densities and melting points than groups 1 & 2. (1 or 2 valence electrons) Lanthanides and Actinides: These are also transition metals that were taken out and placed at the bottom of the table so the table wouldn’t be so wide. The el ...
... and good conductors of heat and electricity. They also have higher densities and melting points than groups 1 & 2. (1 or 2 valence electrons) Lanthanides and Actinides: These are also transition metals that were taken out and placed at the bottom of the table so the table wouldn’t be so wide. The el ...
Science 2nd prep 1st term 1st lesson Many attempts are made by
... *Elements of (A) groups lie on the left and right of the table, you can locate their position in the modern periodic table by knowing their atomic numbers and vice versa. *The elements of (B) groups lie in the middle of the table. *Recently discovered elements are not found in nature but they are pr ...
... *Elements of (A) groups lie on the left and right of the table, you can locate their position in the modern periodic table by knowing their atomic numbers and vice versa. *The elements of (B) groups lie in the middle of the table. *Recently discovered elements are not found in nature but they are pr ...
Page 8: Review 1
... List the early attempts of classification of the elements. Match scientists and their contributions to the development of the P.T. State the modern periodic law. Distinguish groups and periods in the P.T. Define chemical stability using the octet rule. Use the periodic table to predict electron conf ...
... List the early attempts of classification of the elements. Match scientists and their contributions to the development of the P.T. State the modern periodic law. Distinguish groups and periods in the P.T. Define chemical stability using the octet rule. Use the periodic table to predict electron conf ...
C5 Chemicals of the Natural Environment SOW
... Students begin by learning about the substances found in the air, which are molecular, and hence find out about covalent bonding and the properties of simple molecular substances. Next we explore the ionic compounds found in seawater, in particular how the ionic bond forms ionic crystals. Students t ...
... Students begin by learning about the substances found in the air, which are molecular, and hence find out about covalent bonding and the properties of simple molecular substances. Next we explore the ionic compounds found in seawater, in particular how the ionic bond forms ionic crystals. Students t ...
The periodic table and electron structure - Chemistry
... Each group has characteristic properties that are directly related to electron configuration. In going from top to bottom of any group, each element has one more occupied energy level than the element above it. Otherwise, their electron structures are quite similar. As a result, the elements in a ve ...
... Each group has characteristic properties that are directly related to electron configuration. In going from top to bottom of any group, each element has one more occupied energy level than the element above it. Otherwise, their electron structures are quite similar. As a result, the elements in a ve ...
unit 3 ppt
... strongly with water to produce hydrogen gas and aqueous solutions of substances known as alkalis. Because of their extreme reactivity with air or moisture, alkali metals are usually stored in kerosene. Proceeding down the column, the elements of Group 1 melt at successively lower temperatures. ...
... strongly with water to produce hydrogen gas and aqueous solutions of substances known as alkalis. Because of their extreme reactivity with air or moisture, alkali metals are usually stored in kerosene. Proceeding down the column, the elements of Group 1 melt at successively lower temperatures. ...
GENERAL CHARACTERISTICS OF THE p
... and +3 states, respectively indicate that two electrons do not participate in bonding. The reluctance of s-electrons to take part in chemical bonding is known as inert pair effect. The so called “inert pair effect” is therefore, ascribed to two factors. ...
... and +3 states, respectively indicate that two electrons do not participate in bonding. The reluctance of s-electrons to take part in chemical bonding is known as inert pair effect. The so called “inert pair effect” is therefore, ascribed to two factors. ...
d) Ramsay. The idea of arranging the elements in the periodic table
... The most useful source of general information about the elements for anyone associated with chemistry is a a) calculator. ...
... The most useful source of general information about the elements for anyone associated with chemistry is a a) calculator. ...
Chapter 23 Metals and Metallurgy
... • The electron-sea model does not explain observed trends in melting point, boiling point, heat of fusion, etc. – The model suggests these properties should increase with increasing number of valence ...
... • The electron-sea model does not explain observed trends in melting point, boiling point, heat of fusion, etc. – The model suggests these properties should increase with increasing number of valence ...
periodic classification of elements
... It was found that the Law of Octaves was only applicable upto calcium, as after calcium every eighth element did not possess properties similar to the first element. It was assumed by Newland that only 56 elements existed in nature and no more elements would be discovered in the future. But later on ...
... It was found that the Law of Octaves was only applicable upto calcium, as after calcium every eighth element did not possess properties similar to the first element. It was assumed by Newland that only 56 elements existed in nature and no more elements would be discovered in the future. But later on ...
7-1 Notes: Arranging the Elements
... arranged by atomic ___________. In the modern periodic table, elements are arranged _______________ in order of increasing atomic number. Elements that have similar chemical properties are grouped in ___________ columns. Elements are classified according to their ____________ as metals, nonmetals, a ...
... arranged by atomic ___________. In the modern periodic table, elements are arranged _______________ in order of increasing atomic number. Elements that have similar chemical properties are grouped in ___________ columns. Elements are classified according to their ____________ as metals, nonmetals, a ...
Redox
... loses all of its valence electrons. When this occurs it goes from a neutral atom (0 charge) to a cation with a positive charge of: ...
... loses all of its valence electrons. When this occurs it goes from a neutral atom (0 charge) to a cation with a positive charge of: ...
Periodic Classification of Element (NCERT )
... from B to Al. But, Ga has higher ionization enthalpy than Al. Al follows mmediately after s–block elements, whereas Ga follows after d–block elements. The shielding provided by d-electrons is not very effective. These electrons do not shield the valence electrons very effectively. As a result, the v ...
... from B to Al. But, Ga has higher ionization enthalpy than Al. Al follows mmediately after s–block elements, whereas Ga follows after d–block elements. The shielding provided by d-electrons is not very effective. These electrons do not shield the valence electrons very effectively. As a result, the v ...
Periodic Table - Red Deer Public
... usually shown is a compressed view. The Lanthanides and actinides (F block)are cut out and placed at the bottom of the table. ...
... usually shown is a compressed view. The Lanthanides and actinides (F block)are cut out and placed at the bottom of the table. ...
Chem Ch 5 Release Test
... The group of soft, silvery active metals, all of which have one electron in an s orbital, is known as the a. alkaline-earth metals. c. alkali metals. b. transition metals. d. metalloids. The first member of the noble gas family, whose highest energy level consists of an octet of electrons, is a. hel ...
... The group of soft, silvery active metals, all of which have one electron in an s orbital, is known as the a. alkaline-earth metals. c. alkali metals. b. transition metals. d. metalloids. The first member of the noble gas family, whose highest energy level consists of an octet of electrons, is a. hel ...
1.1 elements and the periodic table
... indicate that the element is an alkali metal. If the litmus paper does not turn blue, then the solution is either neutral or possibly acidic. This would indicate that the element is not an alkali metal — other tests could then be carried out to further narrow down which group the element is from. 6. ...
... indicate that the element is an alkali metal. If the litmus paper does not turn blue, then the solution is either neutral or possibly acidic. This would indicate that the element is not an alkali metal — other tests could then be carried out to further narrow down which group the element is from. 6. ...
printer-friendly version of benchmark
... To learn more about Antoine Lavoisier, see http://cti.itc.virginia.edu/~meg3c/classes/tcc313/200Rprojs/lavoisier2/home.html As the discovery of elements and compounds increased over time, so did learning their properties. Due to the overwhelming amount of elements being discovered, chemists needed a ...
... To learn more about Antoine Lavoisier, see http://cti.itc.virginia.edu/~meg3c/classes/tcc313/200Rprojs/lavoisier2/home.html As the discovery of elements and compounds increased over time, so did learning their properties. Due to the overwhelming amount of elements being discovered, chemists needed a ...
Chapter 5
... • In many compounds, the negative charge of the valence electrons is concentrated closer to one atom than to another. • Electronegativity is a measure of the ability of an atom in a chemical compound to attract electrons from another atom in the compound. • Electronegativities tend to increase acros ...
... • In many compounds, the negative charge of the valence electrons is concentrated closer to one atom than to another. • Electronegativity is a measure of the ability of an atom in a chemical compound to attract electrons from another atom in the compound. • Electronegativities tend to increase acros ...
FSN 1500 Week 7 - Oakland Community College
... react with water to form alkaline (basic) solutions; they commonly react with the halogens ...
... react with water to form alkaline (basic) solutions; they commonly react with the halogens ...
Answer
... The first ionization enthalpy of sodium is more than that of magnesium. This is primarily because of two reasons: 1. The atomic size of sodium is greater than that of magnesium 2. The effective nuclear charge of magnesium is higher than that of sodium For these reasons, the energy required to remove ...
... The first ionization enthalpy of sodium is more than that of magnesium. This is primarily because of two reasons: 1. The atomic size of sodium is greater than that of magnesium 2. The effective nuclear charge of magnesium is higher than that of sodium For these reasons, the energy required to remove ...