Snc2d Chapter 5 Practice Test
... 6. a) What are valence electrons? B) Why are they important? C) How can you determine the number of valence electrons for an atom from the periodic table (excluding the transition elements) 7. Draw the Lewis dot diagram for S ...
... 6. a) What are valence electrons? B) Why are they important? C) How can you determine the number of valence electrons for an atom from the periodic table (excluding the transition elements) 7. Draw the Lewis dot diagram for S ...
Grade 11 Chemistry E.. - hrsbstaff.ednet.ns.ca
... g. Na2SO4(aq) + BaCl2(aq) → BaSO4(s) + NaCl(aq) h. CH3OH(l) + O2(g) → CO2(g) + H2O(g) 25. Classify each of the above according to the 5 types of reactions (composition, decomposition, single replacement, double replacement and combustion). 26. Write the formula for each material correctly and then b ...
... g. Na2SO4(aq) + BaCl2(aq) → BaSO4(s) + NaCl(aq) h. CH3OH(l) + O2(g) → CO2(g) + H2O(g) 25. Classify each of the above according to the 5 types of reactions (composition, decomposition, single replacement, double replacement and combustion). 26. Write the formula for each material correctly and then b ...
Reading the Periodic Table
... •Have properties of both metals and nonmetals. •Some of the metalloids, such as silicon and germanium, are semi-conductors. This means that they can carry an electrical charge under special conditions. This property makes metalloids useful in computers and calculators ...
... •Have properties of both metals and nonmetals. •Some of the metalloids, such as silicon and germanium, are semi-conductors. This means that they can carry an electrical charge under special conditions. This property makes metalloids useful in computers and calculators ...
Topic 4: Classifying Elements What did the early chemists use to
... They are called periods, and there are 7 of them. What is the VERTICAL COLUMN in a periodic table called (it can have two names)? How many of these are there in the periodic table? The ...
... They are called periods, and there are 7 of them. What is the VERTICAL COLUMN in a periodic table called (it can have two names)? How many of these are there in the periodic table? The ...
C3 The Periodic Table
... order by mass and saw similarities between every 8th element . • Mendeleev ordered the elements based on their atomic weights and arranged them so there was a pattern. • He left GAPS because he predicted new elements would fit in, as they were discovered. He was right! ...
... order by mass and saw similarities between every 8th element . • Mendeleev ordered the elements based on their atomic weights and arranged them so there was a pattern. • He left GAPS because he predicted new elements would fit in, as they were discovered. He was right! ...
2- Periodic Trends
... Columns of the periodic table are called groups. •Elements in groups have similar properties A family is a group with a specific name: Family names and locations need to be memorized GROUP ...
... Columns of the periodic table are called groups. •Elements in groups have similar properties A family is a group with a specific name: Family names and locations need to be memorized GROUP ...
The Periodic Table
... elements in order of increasing atomic number. • The Atomic number is the number of protons in the nucleus of an atom. ...
... elements in order of increasing atomic number. • The Atomic number is the number of protons in the nucleus of an atom. ...
HOMEWORK : CHAPTER 20
... 20.34 Starting with magnesium and concentrated nitric acid, describe how you would prepare magnesium oxide. [Hint : First convert Mg to Mg(NO3)2. Next, MgO can be obtained by heating Mg(NO3)2] 20.36 The second ionization energy of magnesium is only about twice as great as the first, but the third io ...
... 20.34 Starting with magnesium and concentrated nitric acid, describe how you would prepare magnesium oxide. [Hint : First convert Mg to Mg(NO3)2. Next, MgO can be obtained by heating Mg(NO3)2] 20.36 The second ionization energy of magnesium is only about twice as great as the first, but the third io ...
Name: Date: AP Chemistry/Chemistry 145 Summer Assignment
... purified iron. The other product of the reaction is carbon dioxide gas. 2.10 g of iron is recovered from one such trial. ...
... purified iron. The other product of the reaction is carbon dioxide gas. 2.10 g of iron is recovered from one such trial. ...
CHE 1401 - Fall 2013 - Chapter 7 Homework 7 (Chapter 7: Periodic
... 1. It is not really a member of any particular group. 2. Its electron is not at all shielded from its nucleus. 3. It is the lightest element. 4. It is the only element to exist at room temperature as a diatomic gas. 5. It exhibits some chemical properties similar to those of groups 1A and 7A. A) 1, ...
... 1. It is not really a member of any particular group. 2. Its electron is not at all shielded from its nucleus. 3. It is the lightest element. 4. It is the only element to exist at room temperature as a diatomic gas. 5. It exhibits some chemical properties similar to those of groups 1A and 7A. A) 1, ...
assignment-07-a3
... (Metallic character increases toward the bottom left side of the periodic table.) 15- Which of the following is not a property of an alkali metal? a) Alkali metals are highly reactive towards oxygen and water. b) Alkali metals can be cut with a dull knife. c) Alkali metals have relatively low meltin ...
... (Metallic character increases toward the bottom left side of the periodic table.) 15- Which of the following is not a property of an alkali metal? a) Alkali metals are highly reactive towards oxygen and water. b) Alkali metals can be cut with a dull knife. c) Alkali metals have relatively low meltin ...
Reactions of common metals and properties of
... whereas all the others are metallic solids. In some ways the chemistry of the alkali metal cations resembles that of the proton, H+, but there are more differences than similarities. Hydrogen also forms an anion, the hydride ion, H-, and many metals, including the alkali and alkaline earth metals fo ...
... whereas all the others are metallic solids. In some ways the chemistry of the alkali metal cations resembles that of the proton, H+, but there are more differences than similarities. Hydrogen also forms an anion, the hydride ion, H-, and many metals, including the alkali and alkaline earth metals fo ...
Name: ______ Aim 36: Elements, atoms, compounds and miztures
... All elements retain their original properties. New properties are formed. Only metals retain their original properties. New elements are formed. ...
... All elements retain their original properties. New properties are formed. Only metals retain their original properties. New elements are formed. ...
Review Packet
... 28. Hugh was born 6.391875 X 103 days ago. How old (in years, with 1yr= 365.25 days) is Hugh? ...
... 28. Hugh was born 6.391875 X 103 days ago. How old (in years, with 1yr= 365.25 days) is Hugh? ...
Name: Chemistry A Date: Period: Unit 1 Test Review Packet
... 7. Draw the Bohr electron configuration for phosphorus (before it satisfies the Octet Rule). ...
... 7. Draw the Bohr electron configuration for phosphorus (before it satisfies the Octet Rule). ...
UNIT 1 - MATTER AND CHEMICAL BONDING
... f) ferrous iodide l) cobalt(III) sulphate 5. Classify each of the following reactions as synthesis, single displacement, double displacement, combustion or decomposition. a) iron + copper(I) nitrate iron(II) nitrate + copper b) phosphorus + oxygen diphosphorus pentoxide c) calcium carbonate ca ...
... f) ferrous iodide l) cobalt(III) sulphate 5. Classify each of the following reactions as synthesis, single displacement, double displacement, combustion or decomposition. a) iron + copper(I) nitrate iron(II) nitrate + copper b) phosphorus + oxygen diphosphorus pentoxide c) calcium carbonate ca ...
The Periodic Table - Harlan Independent Schools
... batteries, flashbulbs, and special alloys. The lighter alkaline earth metals such as magnesium and calcium are very important in animal and plant physiology. You all know that calcium helps build your bones. ...
... batteries, flashbulbs, and special alloys. The lighter alkaline earth metals such as magnesium and calcium are very important in animal and plant physiology. You all know that calcium helps build your bones. ...
NAME: Unit 3 Test Review Arsenic (As), Selenium (Se), and
... 20. How is an element in group 1, period 3 similar to an element in group 1 period 5? 21. Have scientist been researchinbg atoms for a long period of time? Yes or No 22. Who was the scientist that discovered that patterns appeared when elements were arranged in order of increasing atomic mass? 23. D ...
... 20. How is an element in group 1, period 3 similar to an element in group 1 period 5? 21. Have scientist been researchinbg atoms for a long period of time? Yes or No 22. Who was the scientist that discovered that patterns appeared when elements were arranged in order of increasing atomic mass? 23. D ...
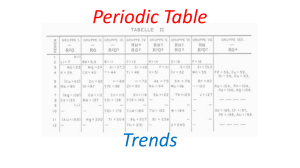
Periodic Table Trends
... reactive it is. The reactivity of the non-metals in Group VII therefore increases as one moves up the group as does their non-metallic nature. Fluorine (F) is the most reactive element and astatine (At) is the least reactive element in Group ...
... reactive it is. The reactivity of the non-metals in Group VII therefore increases as one moves up the group as does their non-metallic nature. Fluorine (F) is the most reactive element and astatine (At) is the least reactive element in Group ...
STUDY GUIDE CHAPTER 8 TEST AND ELEMENT SYMBOLS
... Pure _______________________ are often stored in oil to keep them from reacting with water and oxygen. ALKALI METALS Sodium and potassium are _______________________ metals. ALKALI METALS Chlorine and bromine are examples of _______________________. HALOGENS Semiconductors, also known as ___________ ...
... Pure _______________________ are often stored in oil to keep them from reacting with water and oxygen. ALKALI METALS Sodium and potassium are _______________________ metals. ALKALI METALS Chlorine and bromine are examples of _______________________. HALOGENS Semiconductors, also known as ___________ ...
CI_Chap_1_Test_A_Study_Guide
... Noble gases are not likely to react with any other element. A metalloid is an element that has properties of both metals and non-metals. Metals are easy to shape by drawing into long thin wire and have a shiny surface. Halogens are elements often used to kill bacteria. Transition metals like Gold ar ...
... Noble gases are not likely to react with any other element. A metalloid is an element that has properties of both metals and non-metals. Metals are easy to shape by drawing into long thin wire and have a shiny surface. Halogens are elements often used to kill bacteria. Transition metals like Gold ar ...
+ 2 HCL(aq) CaCl2(aq) + H2O(l) + CO2(g)
... Subscript: A number that represents how many atoms of an element are in a compound. Compound: A substance made of the combined atoms of two or more elements. Chemical Formula: States what elements a compound contains and the exact number of atoms of these elements. Oxidation Number: positive or nega ...
... Subscript: A number that represents how many atoms of an element are in a compound. Compound: A substance made of the combined atoms of two or more elements. Chemical Formula: States what elements a compound contains and the exact number of atoms of these elements. Oxidation Number: positive or nega ...