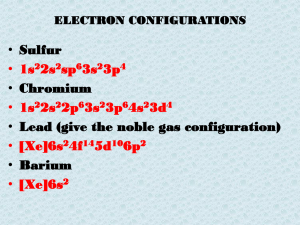
Review of Basic Chemistry
... A chemical group/family is a vertical column of elements that have similar physical and chemical properties. On the periodic table there are 18 vertical groups. Column 1: alkali metals Column 2: alkaline earth metals Column 3-11: transition metals Column 17: halogens Column 18: noble gases/inert gas ...
... A chemical group/family is a vertical column of elements that have similar physical and chemical properties. On the periodic table there are 18 vertical groups. Column 1: alkali metals Column 2: alkaline earth metals Column 3-11: transition metals Column 17: halogens Column 18: noble gases/inert gas ...
File
... nonmagnetic, insulators. Metalloids- properties of both, sometimes called semi-conductors. ...
... nonmagnetic, insulators. Metalloids- properties of both, sometimes called semi-conductors. ...
Writing formulas and naming ionic bonds
... • What is the half-life of an isotope if 125 g of a 1000 g sample of the isotope remains after 3.0 years? • To go from 1000 g to 125 g means that 3 half lives have passed. The half life must be 1 year. 1 year (1 half-life= 500 g) 2 year (2 half-lives = 250 g) 3 years (3 half-lives = 125 g left) ...
... • What is the half-life of an isotope if 125 g of a 1000 g sample of the isotope remains after 3.0 years? • To go from 1000 g to 125 g means that 3 half lives have passed. The half life must be 1 year. 1 year (1 half-life= 500 g) 2 year (2 half-lives = 250 g) 3 years (3 half-lives = 125 g left) ...
Elements and the Periodic Table Section One
... Malleable: a term used to describe material that can be hammered or rolled into shape (pg. ...
... Malleable: a term used to describe material that can be hammered or rolled into shape (pg. ...
Year 11 Chemistry Balancing Equations
... 6. Be 7. Mg 8. Ca 9. Sr 10. C 11. O 12. S 13. F 14. Cl 15. Br 16. I 17. He 18. Ne 19. Ar 20. Kr 21. Xe 22. F ...
... 6. Be 7. Mg 8. Ca 9. Sr 10. C 11. O 12. S 13. F 14. Cl 15. Br 16. I 17. He 18. Ne 19. Ar 20. Kr 21. Xe 22. F ...
Slide 1
... They are never found uncombined in nature. They have two valence electrons. Alkaline earth metals include magnesium and calcium, among others. ...
... They are never found uncombined in nature. They have two valence electrons. Alkaline earth metals include magnesium and calcium, among others. ...
The periodic table is a map of the elements.
... – Sodium and potassium ions, Na+ and K+, are important for life and play an essential role in the functioning of living cells ...
... – Sodium and potassium ions, Na+ and K+, are important for life and play an essential role in the functioning of living cells ...
Chemistry 104: Introduction to the Chemistry of Materials
... penalized 10% per day. No papers will be accepted after 12:45 pm on Monday, September 29. Grammar and Spelling 2 points Although the paper will be only a few paragraphs long, the information should be reported in complete sentences. Please use double spacing. Content 6 points 1. Identify the element ...
... penalized 10% per day. No papers will be accepted after 12:45 pm on Monday, September 29. Grammar and Spelling 2 points Although the paper will be only a few paragraphs long, the information should be reported in complete sentences. Please use double spacing. Content 6 points 1. Identify the element ...
HonorsCh6PracticeTest14
... 12. Which sublevel corresponds to the transition metals in the periodic table? a. s b. p c. d d. f 13. The category of elements that is characterized by the filling of f orbitals is the a. inner transition metals. c. alkali earth metals. b. alkali metals. d. transition metals. 14. The representative ...
... 12. Which sublevel corresponds to the transition metals in the periodic table? a. s b. p c. d d. f 13. The category of elements that is characterized by the filling of f orbitals is the a. inner transition metals. c. alkali earth metals. b. alkali metals. d. transition metals. 14. The representative ...
Compound Name
... Combustion (hydrocarbon combines with oxygen to form carbon dioxide and water) ...
... Combustion (hydrocarbon combines with oxygen to form carbon dioxide and water) ...
honors final key
... c. A barium sulfate precipitate and aqueous sodium chloride are formed from the metathesis reaction between barium chloride and sodium sulfate solutions. BaCl2(aq) + Na2SO4(aq) BaSO4(s) + NaCl(aq) double replacement will occur forms a precipitate ...
... c. A barium sulfate precipitate and aqueous sodium chloride are formed from the metathesis reaction between barium chloride and sodium sulfate solutions. BaCl2(aq) + Na2SO4(aq) BaSO4(s) + NaCl(aq) double replacement will occur forms a precipitate ...
Year 10 Chemistry Exam June 2012
... They are called the noble or inert gases. They are very stable and rarely react. They are also known as Group 18. They all contain eight electrons in their outer shells ...
... They are called the noble or inert gases. They are very stable and rarely react. They are also known as Group 18. They all contain eight electrons in their outer shells ...
Powerpoint - Valence Electrons
... Valence Electrons • Valence electrons are found in the outermost shell. • Most elements in the same family have the same number of valence electrons Examples: Halogens = seven valence electrons Alkali = one valence electron Alkali Earth metals = two valence electrons Noble Gases - are stable and no ...
... Valence Electrons • Valence electrons are found in the outermost shell. • Most elements in the same family have the same number of valence electrons Examples: Halogens = seven valence electrons Alkali = one valence electron Alkali Earth metals = two valence electrons Noble Gases - are stable and no ...
Chapter 5
... Metals • The elements in Group 2A are called alkaline earth metals • All alkaline earth metals have two valence electrons • They are harder than group 1A • Differences in reactivity among the alkaline earth metals are shown by the ways they react with water • Calcium, strontium and barium react easi ...
... Metals • The elements in Group 2A are called alkaline earth metals • All alkaline earth metals have two valence electrons • They are harder than group 1A • Differences in reactivity among the alkaline earth metals are shown by the ways they react with water • Calcium, strontium and barium react easi ...
Standard EPS Shell Presentation
... Remember, the atomic number is the number of protons all atoms of that element have in their nuclei. If the atom is neutral, it will have the same number of electrons as protons. ...
... Remember, the atomic number is the number of protons all atoms of that element have in their nuclei. If the atom is neutral, it will have the same number of electrons as protons. ...
Revision of Chemistry work
... 2. Name 4 other elements not in the first 20 on the periodic table and give their chemical symbol. 3. Name two elements that have been named after planets. 4. Make a word using element symbols. 5. Which are there most of in the periodic table? Solids, Liquids or Gases. 6. Which are there less of in ...
... 2. Name 4 other elements not in the first 20 on the periodic table and give their chemical symbol. 3. Name two elements that have been named after planets. 4. Make a word using element symbols. 5. Which are there most of in the periodic table? Solids, Liquids or Gases. 6. Which are there less of in ...
atomic number
... their atomic mass, and as he did, he found that the families had similar chemical properties. Blank spaces were left open to add the new elements he predicted would occur. ...
... their atomic mass, and as he did, he found that the families had similar chemical properties. Blank spaces were left open to add the new elements he predicted would occur. ...
The Periodic Table PP
... • Never found in nature as “pure elements” but are found as compounds • Lithium, Sodium, Potassium, Rubidium, Cesium, and Francium ...
... • Never found in nature as “pure elements” but are found as compounds • Lithium, Sodium, Potassium, Rubidium, Cesium, and Francium ...
File
... tightly bound to the nucleus (more p+ in the nucleus). Down a group, IE decreases because electrons are farther from the nucleus and not held as tight. Values are found on Table S. ...
... tightly bound to the nucleus (more p+ in the nucleus). Down a group, IE decreases because electrons are farther from the nucleus and not held as tight. Values are found on Table S. ...
Port Said International Schools
... 19. Water volume increases when water converts into ice. Because when the temperature decreases than 4 c the water molecules are collected by hydrogen bonds forming large sized hexagonal crystals with many spaces between them 20. Thermal pollution is very dangerous on marine creatures. Due to separa ...
... 19. Water volume increases when water converts into ice. Because when the temperature decreases than 4 c the water molecules are collected by hydrogen bonds forming large sized hexagonal crystals with many spaces between them 20. Thermal pollution is very dangerous on marine creatures. Due to separa ...