Name
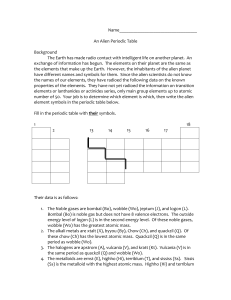
... the names of our elements, they have radioed the following data on the known properties of the elements. They have not yet radioed the information on transition elements or lanthanides or actinides series, only main group elements up to atomic number of 50. Your job is to determine which element is ...
... the names of our elements, they have radioed the following data on the known properties of the elements. They have not yet radioed the information on transition elements or lanthanides or actinides series, only main group elements up to atomic number of 50. Your job is to determine which element is ...
Electron Configurations and the Periodic Table
... •The electron configuration of an atom’s highest occupied energy level generally governs the atom’s chemical properties (the highest occupied level of the noble gases contain stable octets – outer s and p orbitals are completely filled with 8 electrons. •The exception is helium (2 electrons in highe ...
... •The electron configuration of an atom’s highest occupied energy level generally governs the atom’s chemical properties (the highest occupied level of the noble gases contain stable octets – outer s and p orbitals are completely filled with 8 electrons. •The exception is helium (2 electrons in highe ...
Periodic trends Tempura
... Mendeleev said that the properties of the elements are periodic if elements are arranged by increasing atomic mass. The use of mass was incorrect as Mendeleev found with the discovery of reversed pairs. Modern periodic law says the properties are periodic (and elements are in the same column if they ...
... Mendeleev said that the properties of the elements are periodic if elements are arranged by increasing atomic mass. The use of mass was incorrect as Mendeleev found with the discovery of reversed pairs. Modern periodic law says the properties are periodic (and elements are in the same column if they ...
The Periodic Table
... Don’t match the properties of any groups Hydrogen belongs to a family of its own. Most abundant element in the universe Hydrogen is a diatomic, reactive gas. Hydrogen reacts violently with oxygen. The hot water vapor that forms as a result pushed the space shuttle into orbit. Placed above the group ...
... Don’t match the properties of any groups Hydrogen belongs to a family of its own. Most abundant element in the universe Hydrogen is a diatomic, reactive gas. Hydrogen reacts violently with oxygen. The hot water vapor that forms as a result pushed the space shuttle into orbit. Placed above the group ...
Chemistry Unit Review
... b. When sugar (C12H22O11) and sulfuric acid (H2SO4) are combined, carbon, water, and sulfur dioxide are formed. ...
... b. When sugar (C12H22O11) and sulfuric acid (H2SO4) are combined, carbon, water, and sulfur dioxide are formed. ...
UNIT 3 –TEST REVIEW 1 Atoms of which of the
... According to the Periodic Table, which of the following is the MOST accurate model of an atom of Boron? *B has 3 v.e’s (in Group 13), 5 P and 6 N ...
... According to the Periodic Table, which of the following is the MOST accurate model of an atom of Boron? *B has 3 v.e’s (in Group 13), 5 P and 6 N ...
DRILLING FLUID PRODUCTS - CALCIUM CHLORIDE PRODUCT
... Calcium Chloride is used as a soluble weighting agent for water base fluids. This product may also be used in oil based and synthetic drilling fluids. RECOMMENDED TREATMENT Calcium Chloride should be mixed through the hopper. Application rates will depend on drilling and fluid conditions. PRODUCT AD ...
... Calcium Chloride is used as a soluble weighting agent for water base fluids. This product may also be used in oil based and synthetic drilling fluids. RECOMMENDED TREATMENT Calcium Chloride should be mixed through the hopper. Application rates will depend on drilling and fluid conditions. PRODUCT AD ...
Notes
... Maintaining a concentration gradient across a cell wall requires a pumping system, with the energy supplied by ATP. For instance, the Na+/K+-ATPase pump which acts like a ‘revolving door’ to transport ions across the cell membrane. Sodium. Na+ is the principal extracellular solute and maintains the ...
... Maintaining a concentration gradient across a cell wall requires a pumping system, with the energy supplied by ATP. For instance, the Na+/K+-ATPase pump which acts like a ‘revolving door’ to transport ions across the cell membrane. Sodium. Na+ is the principal extracellular solute and maintains the ...
The Chinese High School
... More ACE ideas to think about: If metals can be easily flattened into sheets and drawn into wires, why are our metal pots and pans so hard? What is steel? It cannot be found in the periodic table – so it is not an element! On the same note, what is brass? Or bronze? What is 12K/18K/24K gold? What is ...
... More ACE ideas to think about: If metals can be easily flattened into sheets and drawn into wires, why are our metal pots and pans so hard? What is steel? It cannot be found in the periodic table – so it is not an element! On the same note, what is brass? Or bronze? What is 12K/18K/24K gold? What is ...
Chemical Reactions
... Metals are found in the Earth’s crust, usually combined with other elements, as ores. Metals are extracted from their ores by different methods. Gold Gold is found uncombined in the earth’s crust. The pure metal is separated by panning. Silver and mercury Silver and mercury are found as ores (silver ...
... Metals are found in the Earth’s crust, usually combined with other elements, as ores. Metals are extracted from their ores by different methods. Gold Gold is found uncombined in the earth’s crust. The pure metal is separated by panning. Silver and mercury Silver and mercury are found as ores (silver ...
How is the periodic table organized?
... protons all atoms of that element have in their nuclei. If the atom is neutral, it will have the same number of electrons as protons. ...
... protons all atoms of that element have in their nuclei. If the atom is neutral, it will have the same number of electrons as protons. ...
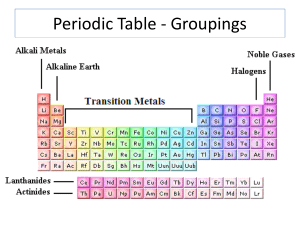
Families of Elements
... Elements in group IA of the periodic table, with the exception of hydrogen Have one electron in their outer energy levels Are the most chemically active of all metals (meaning an element readily combines with other substances to form compounds) NEVER found in pure form A way to identify al ...
... Elements in group IA of the periodic table, with the exception of hydrogen Have one electron in their outer energy levels Are the most chemically active of all metals (meaning an element readily combines with other substances to form compounds) NEVER found in pure form A way to identify al ...
Periods
... Group 8 = 8 electrons Except for He, it has 2 electrons Each column is called a “group” or family. Each element in a group has the same number of electrons in its outer orbital, also known as its outer shell. The electrons in the outer shell are called “valence electrons.” ...
... Group 8 = 8 electrons Except for He, it has 2 electrons Each column is called a “group” or family. Each element in a group has the same number of electrons in its outer orbital, also known as its outer shell. The electrons in the outer shell are called “valence electrons.” ...
Mr. Trachtenberg`s Big Chemistry Test Review Part I of III 20pts
... 11.) Elements in the upper right-‐hand corner of the Periodic Table have the highest electronegativies and ionization energies. 12.) Moving down a group atoms get larger 13.) Moving from l ...
... 11.) Elements in the upper right-‐hand corner of the Periodic Table have the highest electronegativies and ionization energies. 12.) Moving down a group atoms get larger 13.) Moving from l ...
Chemistry 1st Semester Practice Exam
... B. be part of a homogeneous mixture C. be separated into other substances by chemical means ...
... B. be part of a homogeneous mixture C. be separated into other substances by chemical means ...
Periodic Table Powerpoint
... Noble Gases are colorless gases that are extremely unreactive. One important property of the noble gases is their inactivity. They are inactive because their outermost energy level is full. Because they do not readily combine with other elements to form compounds, the noble gases are called inert. T ...
... Noble Gases are colorless gases that are extremely unreactive. One important property of the noble gases is their inactivity. They are inactive because their outermost energy level is full. Because they do not readily combine with other elements to form compounds, the noble gases are called inert. T ...
Chapter 4
... • They are highly reactive. The will react with both air and water. • They form alkaline/basic solutions (the opposite of acidic solutions). • Their electron configurations all end s1. ...
... • They are highly reactive. The will react with both air and water. • They form alkaline/basic solutions (the opposite of acidic solutions). • Their electron configurations all end s1. ...
The s-Block Elements - GCG-42
... BeCl2 is essentially covalent, with comparatively low m.pt. The lower members in group II form essentially ionic chlorides, with Mg having intermediate properties. ...
... BeCl2 is essentially covalent, with comparatively low m.pt. The lower members in group II form essentially ionic chlorides, with Mg having intermediate properties. ...
Study Guide
... a column in the periodic table; elements in the same family will have similar properties (same as family) ...
... a column in the periodic table; elements in the same family will have similar properties (same as family) ...
Final Exam review semester 1
... 1. Ninety-nine percent of all the matter that can be observed in the universe exists as 2. Hydrochloric acid, HCl, is added to solid NaOH. After the reaction is complete, NaCl dissolved in water remains. What are the products of this chemical reaction? ____ ...
... 1. Ninety-nine percent of all the matter that can be observed in the universe exists as 2. Hydrochloric acid, HCl, is added to solid NaOH. After the reaction is complete, NaCl dissolved in water remains. What are the products of this chemical reaction? ____ ...
Chapter 10
... (lithium, sodium, potassium, rubidium, cesium, and francium) 8. Alkaline-earth metal-one of the elements of Group 2 of the periodic table (beryllium, magnesium, calcium, strontium, barium, and radium) 9. Halogen-one of the elements of Group 17 of the periodic table (fluorine, chlorine, bromine, iodi ...
... (lithium, sodium, potassium, rubidium, cesium, and francium) 8. Alkaline-earth metal-one of the elements of Group 2 of the periodic table (beryllium, magnesium, calcium, strontium, barium, and radium) 9. Halogen-one of the elements of Group 17 of the periodic table (fluorine, chlorine, bromine, iodi ...
Periodic Table
... • All atoms what to have a balanced charge but they also want to have a full valance shell. Atoms will often take, loose or share electrons on order to fill the valance. All atoms that have the same # of electrons behave in a similar fashion. The atoms with fewer electrons on the valance and/or the ...
... • All atoms what to have a balanced charge but they also want to have a full valance shell. Atoms will often take, loose or share electrons on order to fill the valance. All atoms that have the same # of electrons behave in a similar fashion. The atoms with fewer electrons on the valance and/or the ...
Chapter 6 Periodic law- states that when the elements are arranged
... periodic repetition of their chemical and physical properties Group- A vertical column of elements in the periodic table; also called a family Period- A horizontal row of elements in the modern periodic table Representative element- groups of elements in the modern periodic table that are designated ...
... periodic repetition of their chemical and physical properties Group- A vertical column of elements in the periodic table; also called a family Period- A horizontal row of elements in the modern periodic table Representative element- groups of elements in the modern periodic table that are designated ...