JP`s Cryptococcus Mini Prep Protocol
... 3. Resuspend cells in 0.5 ml of Protoplasting buffer. Incubate @ 37C for 2 hrs. 4. Collect protoplasts by centrifuging at 5,000 rpm for 5 min. Pour off supernatant under fume hood (pour into chemical waste container) 5. Add 0.5 ml Lysing buffer to each sample. Vortex. Incubate @ 65C for 30 min. 6. W ...
... 3. Resuspend cells in 0.5 ml of Protoplasting buffer. Incubate @ 37C for 2 hrs. 4. Collect protoplasts by centrifuging at 5,000 rpm for 5 min. Pour off supernatant under fume hood (pour into chemical waste container) 5. Add 0.5 ml Lysing buffer to each sample. Vortex. Incubate @ 65C for 30 min. 6. W ...
File
... Maxwell–Boltzmann distribution: The Maxwell–Boltzmann distribution or Maxwell speed distribution describes particle speeds in idealized gases where the particles move freely inside a stationary container without interacting with one another, except for very brief collisions in which they exchange en ...
... Maxwell–Boltzmann distribution: The Maxwell–Boltzmann distribution or Maxwell speed distribution describes particle speeds in idealized gases where the particles move freely inside a stationary container without interacting with one another, except for very brief collisions in which they exchange en ...
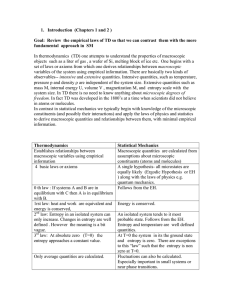
1. Introduction (Chapters 1 and 2 ) Goal: Review the empirical laws
... Consider a system A which is isolated from its surroundings. It is in thermal equilibrium if no observable changes occur. Of course this is an idealization since no system is truly isolated and time independent. The universe and everything in it is constantly changing on some time scale. Two systems ...
... Consider a system A which is isolated from its surroundings. It is in thermal equilibrium if no observable changes occur. Of course this is an idealization since no system is truly isolated and time independent. The universe and everything in it is constantly changing on some time scale. Two systems ...
Pressure Distribution in Uniform Linear Acceleration
... 4.4 Pressure Distribution in Rotating Flows Situations in which a fluid rotates as a solid body are found in many engineering applications. One common application is the centrifugal separator. The centripetal accelerations resulting from rotating a fluid separate the heavier elements from the light ...
... 4.4 Pressure Distribution in Rotating Flows Situations in which a fluid rotates as a solid body are found in many engineering applications. One common application is the centrifugal separator. The centripetal accelerations resulting from rotating a fluid separate the heavier elements from the light ...
module 2
... In each equation the dependent variable, T, is a function of 4 independent variables, (x,y,z,τ); (r, ,z,τ); (r,φ,θ,τ) and is a 2nd order, partial differential equation. The solution of such equations will normally require a numerical solution. For the present, we shall simply look at the simplific ...
... In each equation the dependent variable, T, is a function of 4 independent variables, (x,y,z,τ); (r, ,z,τ); (r,φ,θ,τ) and is a 2nd order, partial differential equation. The solution of such equations will normally require a numerical solution. For the present, we shall simply look at the simplific ...
experimental evaluation of heat exchange between water surface
... heat exchange between water surface and the atmosphere. Another problem is the determination of the equilibrium temperature, because generally the actual water temperature is not equal to the equilibrium temperature. There may occur large differences between actual temperatures and equilibrium tempe ...
... heat exchange between water surface and the atmosphere. Another problem is the determination of the equilibrium temperature, because generally the actual water temperature is not equal to the equilibrium temperature. There may occur large differences between actual temperatures and equilibrium tempe ...
Kidney 1
... limb of the Loop, it passes into a part of the tubule that is much more permeable to salt than to water. NaCl diffuses out of the tubule, leaving water behind. • As the tubular fluid passes through the thick ascending limb, Na+, Cl- and K+ are actively reabsorbed by the NK2C cotransporter. • The tub ...
... limb of the Loop, it passes into a part of the tubule that is much more permeable to salt than to water. NaCl diffuses out of the tubule, leaving water behind. • As the tubular fluid passes through the thick ascending limb, Na+, Cl- and K+ are actively reabsorbed by the NK2C cotransporter. • The tub ...
v = Y
... reservoir when heat is discarded into it (TC). ◦ Any finite temperature drop would result in an irreversible processes. ◦ Every process that involves heat transfer must be isothermal. ◦ Any process in which the the working substance is between TH and TC, there must be no heat transfer into the hot o ...
... reservoir when heat is discarded into it (TC). ◦ Any finite temperature drop would result in an irreversible processes. ◦ Every process that involves heat transfer must be isothermal. ◦ Any process in which the the working substance is between TH and TC, there must be no heat transfer into the hot o ...
Thermodynamics Temperature Scales Thermal Expansion and Stress
... may happen through heat transfer or through mechanical work First law is a statement of conservation of energy Change in internal energy of system equals the difference between the heat added to the system and the work done by the system ΔU = Q − W ...
... may happen through heat transfer or through mechanical work First law is a statement of conservation of energy Change in internal energy of system equals the difference between the heat added to the system and the work done by the system ΔU = Q − W ...
Principles of Technology
... A. The efficiency of a heat engine depends on its operating temperatures. B. A heat engine would reach 100 percent efficiency only if its “cold” temperature were below absolute zero (0 K) such as -150 K. C. Since such an engine cannot be completely efficient, it follows that a temperature of absolut ...
... A. The efficiency of a heat engine depends on its operating temperatures. B. A heat engine would reach 100 percent efficiency only if its “cold” temperature were below absolute zero (0 K) such as -150 K. C. Since such an engine cannot be completely efficient, it follows that a temperature of absolut ...
Answer Key to Sample Questions
... positive because one molecule breaks to form two molecules b. What is the sign of H for this reaction? positive because a bond is broken, but none is formed. c. In which temperature range will this reaction be thermodynamically favored? It is entropy favored, enthalpy disfavored, so favored overall ...
... positive because one molecule breaks to form two molecules b. What is the sign of H for this reaction? positive because a bond is broken, but none is formed. c. In which temperature range will this reaction be thermodynamically favored? It is entropy favored, enthalpy disfavored, so favored overall ...
Physical Chemistry
... The semi-permeable membrane used in reverse osmosis contains tiny pores through which water can flow. The small pores of this membrane are restrictive to such organic compounds as salt and other natural minerals, which generally have a larger molecular composition than water. These pores are also re ...
... The semi-permeable membrane used in reverse osmosis contains tiny pores through which water can flow. The small pores of this membrane are restrictive to such organic compounds as salt and other natural minerals, which generally have a larger molecular composition than water. These pores are also re ...
Countercurrent exchange

Countercurrent exchange is a mechanism occurring in nature and mimicked in industry and engineering, in which there is a crossover of some property, usually heat or some component, between two flowing bodies flowing in opposite directions to each other. The flowing bodies can be liquids, gases, or even solid powders, or any combination of those. For example, in a distillation column, the vapors bubble up through the downward flowing liquid while exchanging both heat and mass.The maximum amount of heat or mass transfer that can be obtained is higher with countercurrent than co-current (parallel) exchange because countercurrent maintains a slowly declining difference or gradient (usually temperature or concentration difference). In cocurrent exchange the initial gradient is higher but falls off quickly, leading to wasted potential. For example, in the diagram at the right, the fluid being heated (exiting top) has a higher exiting temperature than the cooled fluid (exiting bottom) that was used for heating. With cocurrent or parallel exchange the heated and cooled fluids can only approach one another. The result is that countercurrent exchange can achieve a greater amount of heat or mass transfer than parallel under otherwise similar conditions. See: flow arrangement.Countercurrent exchange when set up in a circuit or loop can be used for building up concentrations, heat, or other properties of flowing liquids. Specifically when set up in a loop with a buffering liquid between the incoming and outgoing fluid running in a circuit, and with active transport pumps on the outgoing fluid's tubes, the system is called a Countercurrent multiplier, enabling a multiplied effect of many small pumps to gradually build up a large concentration in the buffer liquid.Other countercurrent exchange circuits where the incoming and outgoing fluids touch each other are used for retaining a high concentration of a dissolved substance or for retaining heat, or for allowing the external buildup of the heat or concentration at one point in the system.Countercurrent exchange circuits or loops are found extensively in nature, specifically in biologic systems. In vertebrates, they are called a Rete mirabile, originally the name of an organ in fish gills for absorbing oxygen from the water. It is mimicked in industrial systems. Countercurrent exchange is a key concept in chemical engineering thermodynamics and manufacturing processes, for example in extracting sucrose from sugar beet roots.Countercurrent multiplication is a similar but different concept where liquid moves in a loop followed by a long length of movement in opposite directions with an intermediate zone. The tube leading to the loop passively building up a gradient of heat (or cooling) or solvent concentration while the returning tube has a constant small pumping action all along it, so that a gradual intensification of the heat or concentration is created towards the loop. Countercurrent multiplication has been found in the kidneys as well as in many other biological organs.