SMP Quiz Session 1
... 4. Some black holes stars exploded. 5. These elements were made by radioacIvity in the Earth's core. ...
... 4. Some black holes stars exploded. 5. These elements were made by radioacIvity in the Earth's core. ...
Topic 7 Atomic and Nuclear Physics
... Nucleon number (A) The number of protons plus neutrons in a nucleus Proton number (Z): The number of protons in a nucleus. Neutron number (N): The number of neutrons in a nucleus. Radioactive half-life: The time taken for half the original nuclei in a sample to decay. Artificial (induced) transmutat ...
... Nucleon number (A) The number of protons plus neutrons in a nucleus Proton number (Z): The number of protons in a nucleus. Neutron number (N): The number of neutrons in a nucleus. Radioactive half-life: The time taken for half the original nuclei in a sample to decay. Artificial (induced) transmutat ...
Nuclear Reactions - Kelso High School
... 66. Here is a list of atomic numbers: a) 6 b) 25 c) 47 d) 80 e) 86 f) 92. Use a periodic table to identify the corresponding elements. 67. For each of the isotopes below state: a) the number of protons b) the number of neutrons. ...
... 66. Here is a list of atomic numbers: a) 6 b) 25 c) 47 d) 80 e) 86 f) 92. Use a periodic table to identify the corresponding elements. 67. For each of the isotopes below state: a) the number of protons b) the number of neutrons. ...
Radioactivity
... one proton (i.e., anything but hydrogen) will have repulsions between the protons in the nucleus. • A strong nuclear force helps keep the nucleus together • Neutrons play a key role stabilizing the nucleus. • Therefore, the ratio of neutrons to protons is an ...
... one proton (i.e., anything but hydrogen) will have repulsions between the protons in the nucleus. • A strong nuclear force helps keep the nucleus together • Neutrons play a key role stabilizing the nucleus. • Therefore, the ratio of neutrons to protons is an ...
Ch 10 Nuclear Chemistry
... • Fusion is a process in which the nuclei of two atoms combine to form a larger nucleus. • During fusion a small fraction of the reactant mass is converted into energy. • Inside the sun an estimated 600 millions tons of hydrogen undergo fusion each second ...
... • Fusion is a process in which the nuclei of two atoms combine to form a larger nucleus. • During fusion a small fraction of the reactant mass is converted into energy. • Inside the sun an estimated 600 millions tons of hydrogen undergo fusion each second ...
Nuclear Reactions - Socastee High School
... similar to the one dropped on Nagasaki in 1945. The energy release at this stage is mainly due to nuclear fission -- because the atoms of plutonium are split. ...
... similar to the one dropped on Nagasaki in 1945. The energy release at this stage is mainly due to nuclear fission -- because the atoms of plutonium are split. ...
Nuclear Notes Introduction
... Radioactivity: is the act of emitting radiation spontaneously with the resulting emission of radiation resulting in the formation of a new nuclei. a. Does not need a source to travel through space and penetrate another material ...
... Radioactivity: is the act of emitting radiation spontaneously with the resulting emission of radiation resulting in the formation of a new nuclei. a. Does not need a source to travel through space and penetrate another material ...
Nuclear physics α −
... repulse each other). The force that holds the protons and neutrons together in the nucleus is a new so called nuclear or strong interaction. What are the features of the nuclear interaction? 1. it does not depend on charge, charge independent: p-p, n-n, and p-n interactions are the same 2. it is alw ...
... repulse each other). The force that holds the protons and neutrons together in the nucleus is a new so called nuclear or strong interaction. What are the features of the nuclear interaction? 1. it does not depend on charge, charge independent: p-p, n-n, and p-n interactions are the same 2. it is alw ...
File
... 19. What is the name of the process in which the nucleus of an atom of one element is changed into the nucleus of an atom of a different element? A) decomposition C) substitution ...
... 19. What is the name of the process in which the nucleus of an atom of one element is changed into the nucleus of an atom of a different element? A) decomposition C) substitution ...
Chapter1
... To solve these problems, Fermi hypothesized the existence of an essentially undetectable additional particle, the neutrino (ν), that carried away the excess energy and missing spin. The neutrino was eventually detected some 30 years later. Measuring ‘geoneutrinos’ is helping us define the comp ...
... To solve these problems, Fermi hypothesized the existence of an essentially undetectable additional particle, the neutrino (ν), that carried away the excess energy and missing spin. The neutrino was eventually detected some 30 years later. Measuring ‘geoneutrinos’ is helping us define the comp ...
The New Alchemy
... Protons – one of the parts of an atom. Protons have a (+) charge and are found in the nucleus. Neutrons – one of the parts of an atom. Neutrons have no charge and are found in the nucleus. Nucleus – found in the center of an atom. It contains protons and neutrons. Nuclei is the plural of nucleus. Nu ...
... Protons – one of the parts of an atom. Protons have a (+) charge and are found in the nucleus. Neutrons – one of the parts of an atom. Neutrons have no charge and are found in the nucleus. Nucleus – found in the center of an atom. It contains protons and neutrons. Nuclei is the plural of nucleus. Nu ...
Atomic/Nuclear
... The binding energy per nucleon versus atomic number graph peaks at iron. Smaller and larger nuclei have less binding energy per nucleon than iron. If a nucleus with an even atomic number 92 or greater and an odd mass number absorbs a neutron, it can be split into two smaller nuclei with about 3 neut ...
... The binding energy per nucleon versus atomic number graph peaks at iron. Smaller and larger nuclei have less binding energy per nucleon than iron. If a nucleus with an even atomic number 92 or greater and an odd mass number absorbs a neutron, it can be split into two smaller nuclei with about 3 neut ...
NUCLEAR CHEMISTRY
... normally strong enough to hold the protons and neutrons together. However, sometimes the force of repulsion due to the protons having the same charge overcomes the strong nuclear force and the atom breaks apart. ...
... normally strong enough to hold the protons and neutrons together. However, sometimes the force of repulsion due to the protons having the same charge overcomes the strong nuclear force and the atom breaks apart. ...
Independent Study: Nuclear Chemistry
... 1. Define radioactive element, radioactive isotopes and know the following isotopes of hydrogen: protium, deuterium, and tritium. Recognize the isotopes in heavy water. (Radioisotopes) 2. Define: radioactive decay, transmutation, neutron bombardment, fission, and fusion. Identify the transuranium el ...
... 1. Define radioactive element, radioactive isotopes and know the following isotopes of hydrogen: protium, deuterium, and tritium. Recognize the isotopes in heavy water. (Radioisotopes) 2. Define: radioactive decay, transmutation, neutron bombardment, fission, and fusion. Identify the transuranium el ...
Independent Study: Nuclear Chemistry
... 1. Define radioactive element, radioactive isotopes and know the following isotopes of hydrogen: protium, deuterium, and tritium. Recognize the isotopes in heavy water. (Radioisotopes) 2. Define: radioactive decay, transmutation, neutron bombardment, fission, and fusion. Identify the transuranium el ...
... 1. Define radioactive element, radioactive isotopes and know the following isotopes of hydrogen: protium, deuterium, and tritium. Recognize the isotopes in heavy water. (Radioisotopes) 2. Define: radioactive decay, transmutation, neutron bombardment, fission, and fusion. Identify the transuranium el ...
Energy per nucleon
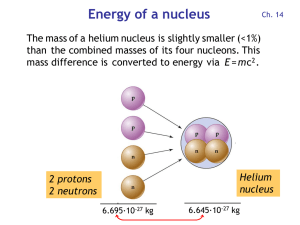
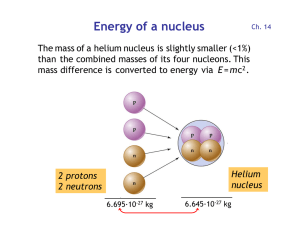
... and 2 neutrons are combined to form the helium nucleus: E = m c2 = (5·10-29 kg) · (3·108 m/s)2 = 4.5 ·10-12 J = 28 MeV Each of the four nucleons releases 28 MeV / 4 = 7 MeV ...
... and 2 neutrons are combined to form the helium nucleus: E = m c2 = (5·10-29 kg) · (3·108 m/s)2 = 4.5 ·10-12 J = 28 MeV Each of the four nucleons releases 28 MeV / 4 = 7 MeV ...
Energy of a nucleus
... • All elements heavier than iron/nickel are created during a supernova explosion, which has enough thermal energy to form nuclei with higher energy per nucleon. ...
... • All elements heavier than iron/nickel are created during a supernova explosion, which has enough thermal energy to form nuclei with higher energy per nucleon. ...
Notes: Nuclear Chemistry
... Fusion = the combining of two atomic nuclei to produce one nucleus of heavier mass. a. Fusion reactions release more energy than fission reactions. b. Fusing nuclei must be brought to a distance less than 10-13cm in order to overcome the strong repulsive forces between two neighboring positively cha ...
... Fusion = the combining of two atomic nuclei to produce one nucleus of heavier mass. a. Fusion reactions release more energy than fission reactions. b. Fusing nuclei must be brought to a distance less than 10-13cm in order to overcome the strong repulsive forces between two neighboring positively cha ...
Word - The Chemistry Book
... b. First to artificially transmute one element into another B. Nuclear reactions 1. Involve great quantities of energy Strong nuclear force overcomes electrostatic repulsion between protons 2. Differ from chemical reactions a. atomic numbers change b. some matter is changed to energy c. specific iso ...
... b. First to artificially transmute one element into another B. Nuclear reactions 1. Involve great quantities of energy Strong nuclear force overcomes electrostatic repulsion between protons 2. Differ from chemical reactions a. atomic numbers change b. some matter is changed to energy c. specific iso ...
Alpha Beta Fission Fusion
... While many elements undergo radioactive decay naturally, nuclear reactions can also be stimulated artificially. Although these reactions also occur naturally, we are most familiar with them as stimulated reactions. There are two such types of nuclear reactions: 1. Nuclear fission: reactions in which ...
... While many elements undergo radioactive decay naturally, nuclear reactions can also be stimulated artificially. Although these reactions also occur naturally, we are most familiar with them as stimulated reactions. There are two such types of nuclear reactions: 1. Nuclear fission: reactions in which ...
- Physics
... each other. If the uranium-235 is in the shape of a sphere about 13 pounds of uranium form a critical mass where a runaway chain reaction (bomb) can occur. I would restate the last paragraph on page 20-4. In the process of fission some mass becomes kinetic energy of the fission fragments. It is true ...
... each other. If the uranium-235 is in the shape of a sphere about 13 pounds of uranium form a critical mass where a runaway chain reaction (bomb) can occur. I would restate the last paragraph on page 20-4. In the process of fission some mass becomes kinetic energy of the fission fragments. It is true ...
Chapter 4: The Structure of the Atom &
... o Above the band of stability – too many _____________; Below the band of stability – too many _______________ or too few ______________ o BETA DECAY: For elements above the band of stability (too many neutrons) A NEUTRON will decay into a PROTON (stays in the nucleus) and an ELECTRON (leaves the ...
... o Above the band of stability – too many _____________; Below the band of stability – too many _______________ or too few ______________ o BETA DECAY: For elements above the band of stability (too many neutrons) A NEUTRON will decay into a PROTON (stays in the nucleus) and an ELECTRON (leaves the ...
nuclear radiation
... ALPHA PARTICLES consists of two protons and two neutrons • Composed of He nucleus atom w/ +2 charge • Least penetrating type of radiation • Can be stopped by a sheet of paper ...
... ALPHA PARTICLES consists of two protons and two neutrons • Composed of He nucleus atom w/ +2 charge • Least penetrating type of radiation • Can be stopped by a sheet of paper ...
Multiple Choice Questions
... (3) a shorter half-life and the same decay mode (4) a shorter half-life and a different decay mode 15. What fraction of a sample of Sr-90 would remain unchanged after 58.2 years? ...
... (3) a shorter half-life and the same decay mode (4) a shorter half-life and a different decay mode 15. What fraction of a sample of Sr-90 would remain unchanged after 58.2 years? ...
Ch 21.1 Study Guide
... 1. _____ Based on the information about the three elementary particles on page 683 of the text, which has the greatest mass? (a) the proton (b) the neutron (c) the electron (d) They all have the same mass. 2. _____ The force that keeps nucleons together is (a) a strong nuclear force. (b) a weak nucl ...
... 1. _____ Based on the information about the three elementary particles on page 683 of the text, which has the greatest mass? (a) the proton (b) the neutron (c) the electron (d) They all have the same mass. 2. _____ The force that keeps nucleons together is (a) a strong nuclear force. (b) a weak nucl ...
Nuclear fission
In nuclear physics and nuclear chemistry, nuclear fission is either a nuclear reaction or a radioactive decay process in which the nucleus of an atom splits into smaller parts (lighter nuclei). The fission process often produces free neutrons and photons (in the form of gamma rays), and releases a very large amount of energy even by the energetic standards of radioactive decay.Nuclear fission of heavy elements was discovered on December 17, 1938 by German Otto Hahn and his assistant Fritz Strassmann, and explained theoretically in January 1939 by Lise Meitner and her nephew Otto Robert Frisch. Frisch named the process by analogy with biological fission of living cells. It is an exothermic reaction which can release large amounts of energy both as electromagnetic radiation and as kinetic energy of the fragments (heating the bulk material where fission takes place). In order for fission to produce energy, the total binding energy of the resulting elements must be less negative (higher energy) than that of the starting element.Fission is a form of nuclear transmutation because the resulting fragments are not the same element as the original atom. The two nuclei produced are most often of comparable but slightly different sizes, typically with a mass ratio of products of about 3 to 2, for common fissile isotopes. Most fissions are binary fissions (producing two charged fragments), but occasionally (2 to 4 times per 1000 events), three positively charged fragments are produced, in a ternary fission. The smallest of these fragments in ternary processes ranges in size from a proton to an argon nucleus.Apart from fission induced by a neutron, harnessed and exploited by humans, a natural form of spontaneous radioactive decay (not requiring a neutron) is also referred to as fission, and occurs especially in very high-mass-number isotopes. Spontaneous fission was discovered in 1940 by Flyorov, Petrzhak and Kurchatov in Moscow, when they decided to confirm that, without bombardment by neutrons, the fission rate of uranium was indeed negligible, as predicted by Niels Bohr; it wasn't.The unpredictable composition of the products (which vary in a broad probabilistic and somewhat chaotic manner) distinguishes fission from purely quantum-tunnelling processes such as proton emission, alpha decay and cluster decay, which give the same products each time. Nuclear fission produces energy for nuclear power and drives the explosion of nuclear weapons. Both uses are possible because certain substances called nuclear fuels undergo fission when struck by fission neutrons, and in turn emit neutrons when they break apart. This makes possible a self-sustaining nuclear chain reaction that releases energy at a controlled rate in a nuclear reactor or at a very rapid uncontrolled rate in a nuclear weapon.The amount of free energy contained in nuclear fuel is millions of times the amount of free energy contained in a similar mass of chemical fuel such as gasoline, making nuclear fission a very dense source of energy. The products of nuclear fission, however, are on average far more radioactive than the heavy elements which are normally fissioned as fuel, and remain so for significant amounts of time, giving rise to a nuclear waste problem. Concerns over nuclear waste accumulation and over the destructive potential of nuclear weapons may counterbalance the desirable qualities of fission as an energy source, and give rise to ongoing political debate over nuclear power.