Basics of Nuclear Physics and Fission
... Beta decay, which the emission of an electron or a positron (a particle identical to an electron except that it has a positive electrical charge). Electron capture, which is the capture by the nucleus of an electron from among the ones whirling around it. In effect, the electron combines with a prot ...
... Beta decay, which the emission of an electron or a positron (a particle identical to an electron except that it has a positive electrical charge). Electron capture, which is the capture by the nucleus of an electron from among the ones whirling around it. In effect, the electron combines with a prot ...
1 AP Chemistry Chapter 21 - The Nucleus: A Chemist`s View 21.1
... 4. Electron Capture a. Inner orbital electron is captured by the nucleus of its own atom b. Electron combines with a proton and a neutron is formed ...
... 4. Electron Capture a. Inner orbital electron is captured by the nucleus of its own atom b. Electron combines with a proton and a neutron is formed ...
Chapter 18 Notes
... 4. Electron Capture a. Inner orbital electron is captured by the nucleus of its own atom b. Electron combines with a proton and a neutron is formed ...
... 4. Electron Capture a. Inner orbital electron is captured by the nucleus of its own atom b. Electron combines with a proton and a neutron is formed ...
Nuclear Physics SL - Hockerill Students
... probably empty space as most of alpha particles go through the foil unchanged ...
... probably empty space as most of alpha particles go through the foil unchanged ...
FUSION AND FISSION
... • Large amount of energy generated – 1 million times more than chemical reactions – Nuclear fusion on the sun – Nuclear fission for reactors ...
... • Large amount of energy generated – 1 million times more than chemical reactions – Nuclear fusion on the sun – Nuclear fission for reactors ...
GRAMMAR: verb tenses
... roughly the same number of neutrons and protons are needed for light nuclei, while for heavier nuclei a few more neutrons are needed (about half again as many neutrons as protons for the heaviest .nuclides, such as U-238). One frequently encounters nuclides which, although unstable, will hang togeth ...
... roughly the same number of neutrons and protons are needed for light nuclei, while for heavier nuclei a few more neutrons are needed (about half again as many neutrons as protons for the heaviest .nuclides, such as U-238). One frequently encounters nuclides which, although unstable, will hang togeth ...
Chapter 3
... As the water boils, heat from the hot stove burner and pan radiates into the surrounding ...
... As the water boils, heat from the hot stove burner and pan radiates into the surrounding ...
APES-Chapter-3
... As the water boils, heat from the hot stove burner and pan radiates into the surrounding ...
... As the water boils, heat from the hot stove burner and pan radiates into the surrounding ...
ib atomic and nuclear physics definitions and concepts
... RADIOACTIVE DECAY: A random and spontaneous process in which unstable nuclei emit a particle (disintegrate). The rate decreases exponentially with time. NATURAL RADIOACTIVE DECAY: The emission of an alpha or beta particle. NUCLEAR STRONG FORCE: The force that holds the particles of a nucleus togethe ...
... RADIOACTIVE DECAY: A random and spontaneous process in which unstable nuclei emit a particle (disintegrate). The rate decreases exponentially with time. NATURAL RADIOACTIVE DECAY: The emission of an alpha or beta particle. NUCLEAR STRONG FORCE: The force that holds the particles of a nucleus togethe ...
Types of Radiation
... Students know how to relate the position of an element in the periodic table to its atomic number and atomic mass. (1a) Students know the energy release per gram of material is much larger in nuclear fusion or fission reactions than in chemical reactions. The change in mass (calculated by E = mc ...
... Students know how to relate the position of an element in the periodic table to its atomic number and atomic mass. (1a) Students know the energy release per gram of material is much larger in nuclear fusion or fission reactions than in chemical reactions. The change in mass (calculated by E = mc ...
Chapter 7 Worksheet
... C Fission reactions involve a heavy unstable nucleus splitting apart to form 2 smaller nuclei, while fusion reactions involve combining two lightweight nuclei to form a heavier nucleus C Fission reactions often produce daughter products that are also radioactive, while fission reactions often do not ...
... C Fission reactions involve a heavy unstable nucleus splitting apart to form 2 smaller nuclei, while fusion reactions involve combining two lightweight nuclei to form a heavier nucleus C Fission reactions often produce daughter products that are also radioactive, while fission reactions often do not ...
Atomic and Nuclear Physics
... • Nucleus of Uranium-235 splits by collision with a neutron to produce 2 daughter nuclei and a small number of neutrons (3) • This process releases energy in the form of kinetic energy (= thermal energy) of the 2 nuclei (fission products) • The neutrons produced by one fission can strike other U-235 ...
... • Nucleus of Uranium-235 splits by collision with a neutron to produce 2 daughter nuclei and a small number of neutrons (3) • This process releases energy in the form of kinetic energy (= thermal energy) of the 2 nuclei (fission products) • The neutrons produced by one fission can strike other U-235 ...
Chapter 19 Radioactive Material An Isotope is an element with a
... uranium atoms split. The hot water turns into steam which then spins a turbine that is hooked u to a generator. To prevent the uranium from creating to much heat that might melt the walls ...
... uranium atoms split. The hot water turns into steam which then spins a turbine that is hooked u to a generator. To prevent the uranium from creating to much heat that might melt the walls ...
Chapter 21 - Richsingiser.com
... • Nuclear fission forms two nuclei of roughly similar size from a single heavy nucleus. • Fission reactions release very large quantities of energy (“exergonic”) and produce several neutrons in addition to two nuclides. ...
... • Nuclear fission forms two nuclei of roughly similar size from a single heavy nucleus. • Fission reactions release very large quantities of energy (“exergonic”) and produce several neutrons in addition to two nuclides. ...
Outline Chapter 8 The Nucleus 8-1. J.J. Thompson`s Plum Pudding
... total binding energy of the nucleus by the number of nucleons (protons and neutrons) it contains; the greater the binding energy per nucleon, the more stable the nucleus. ...
... total binding energy of the nucleus by the number of nucleons (protons and neutrons) it contains; the greater the binding energy per nucleon, the more stable the nucleus. ...
File
... some are beta decay - the stable end point is an element with atomic # less than 83 (lead) - there are also unstable lead isotopes which are intermediates ...
... some are beta decay - the stable end point is an element with atomic # less than 83 (lead) - there are also unstable lead isotopes which are intermediates ...
nuclear chemistry - La Salle High School
... electrons in an atom is captured by the nucleus of the atom ...
... electrons in an atom is captured by the nucleus of the atom ...
solutions - Physicsland
... 9. Gamma radiation produces not only the least change in mass and atomic numbers, but produces no change in mass number, atomic number, or electric charge. Both alpha and beta radiation do produce these changes. 11. The proton “bullets” need enough momentum to overcome the electric force of repulsio ...
... 9. Gamma radiation produces not only the least change in mass and atomic numbers, but produces no change in mass number, atomic number, or electric charge. Both alpha and beta radiation do produce these changes. 11. The proton “bullets” need enough momentum to overcome the electric force of repulsio ...
AC Geophysical Science Study Guide
... 16. Compare the atomic mass of an atom with the atomic mass of the constituent particles that make up that mass. 17. What is binding energy? How do you determine binding energy? 18. The hydrogen isotope H-3 has a nuclear mass of 3.0155 µ, and the helium isotope He-3 has a nuclear mass of 3.0149 µ. F ...
... 16. Compare the atomic mass of an atom with the atomic mass of the constituent particles that make up that mass. 17. What is binding energy? How do you determine binding energy? 18. The hydrogen isotope H-3 has a nuclear mass of 3.0155 µ, and the helium isotope He-3 has a nuclear mass of 3.0149 µ. F ...
nuclear chemistry - Wood County Schools
... and continually split and fuse it to produce energy. Iron (Fe, number 26) has the highest energy stored in its nucleus. Atoms larger than iron will release energy when they undergo fission, and atoms smaller than iron will release energy when they ...
... and continually split and fuse it to produce energy. Iron (Fe, number 26) has the highest energy stored in its nucleus. Atoms larger than iron will release energy when they undergo fission, and atoms smaller than iron will release energy when they ...
Structure of the nucleus • It is now known that the nucleus consists of
... The daughter nucleus produced is Radon-226 (the equation is balanced, giving unknown daughter nucleus atomic number 88 and mass number 226. The periodic table is used to identify the element with atomic number 88 as Radon). ...
... The daughter nucleus produced is Radon-226 (the equation is balanced, giving unknown daughter nucleus atomic number 88 and mass number 226. The periodic table is used to identify the element with atomic number 88 as Radon). ...
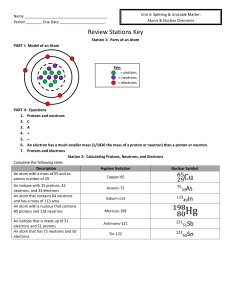
Name Period ______ Due Date Review Stations Key Station 1
... Stopped by paper, wood, cloth, etc. ...
... Stopped by paper, wood, cloth, etc. ...
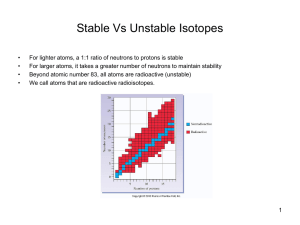
Stable Vs Unstable Isotopes
... For lighter atoms, a 1:1 ratio of neutrons to protons is stable For larger atoms, it takes a greater number of neutrons to maintain stability Beyond atomic number 83, all atoms are radioactive (unstable) We call atoms that are radioactive radioisotopes. ...
... For lighter atoms, a 1:1 ratio of neutrons to protons is stable For larger atoms, it takes a greater number of neutrons to maintain stability Beyond atomic number 83, all atoms are radioactive (unstable) We call atoms that are radioactive radioisotopes. ...
Nuclear Notes
... A crucial factor in the stability of a nucleus is the ratio of neutron number to proton number. Nuclides with more the 20 protons require a larger number of neutrons than protons to moderate the effect of increasing proton repulsions. (Nuclides with less that 20 protons tend to have an equal number ...
... A crucial factor in the stability of a nucleus is the ratio of neutron number to proton number. Nuclides with more the 20 protons require a larger number of neutrons than protons to moderate the effect of increasing proton repulsions. (Nuclides with less that 20 protons tend to have an equal number ...
Nuclear fission
In nuclear physics and nuclear chemistry, nuclear fission is either a nuclear reaction or a radioactive decay process in which the nucleus of an atom splits into smaller parts (lighter nuclei). The fission process often produces free neutrons and photons (in the form of gamma rays), and releases a very large amount of energy even by the energetic standards of radioactive decay.Nuclear fission of heavy elements was discovered on December 17, 1938 by German Otto Hahn and his assistant Fritz Strassmann, and explained theoretically in January 1939 by Lise Meitner and her nephew Otto Robert Frisch. Frisch named the process by analogy with biological fission of living cells. It is an exothermic reaction which can release large amounts of energy both as electromagnetic radiation and as kinetic energy of the fragments (heating the bulk material where fission takes place). In order for fission to produce energy, the total binding energy of the resulting elements must be less negative (higher energy) than that of the starting element.Fission is a form of nuclear transmutation because the resulting fragments are not the same element as the original atom. The two nuclei produced are most often of comparable but slightly different sizes, typically with a mass ratio of products of about 3 to 2, for common fissile isotopes. Most fissions are binary fissions (producing two charged fragments), but occasionally (2 to 4 times per 1000 events), three positively charged fragments are produced, in a ternary fission. The smallest of these fragments in ternary processes ranges in size from a proton to an argon nucleus.Apart from fission induced by a neutron, harnessed and exploited by humans, a natural form of spontaneous radioactive decay (not requiring a neutron) is also referred to as fission, and occurs especially in very high-mass-number isotopes. Spontaneous fission was discovered in 1940 by Flyorov, Petrzhak and Kurchatov in Moscow, when they decided to confirm that, without bombardment by neutrons, the fission rate of uranium was indeed negligible, as predicted by Niels Bohr; it wasn't.The unpredictable composition of the products (which vary in a broad probabilistic and somewhat chaotic manner) distinguishes fission from purely quantum-tunnelling processes such as proton emission, alpha decay and cluster decay, which give the same products each time. Nuclear fission produces energy for nuclear power and drives the explosion of nuclear weapons. Both uses are possible because certain substances called nuclear fuels undergo fission when struck by fission neutrons, and in turn emit neutrons when they break apart. This makes possible a self-sustaining nuclear chain reaction that releases energy at a controlled rate in a nuclear reactor or at a very rapid uncontrolled rate in a nuclear weapon.The amount of free energy contained in nuclear fuel is millions of times the amount of free energy contained in a similar mass of chemical fuel such as gasoline, making nuclear fission a very dense source of energy. The products of nuclear fission, however, are on average far more radioactive than the heavy elements which are normally fissioned as fuel, and remain so for significant amounts of time, giving rise to a nuclear waste problem. Concerns over nuclear waste accumulation and over the destructive potential of nuclear weapons may counterbalance the desirable qualities of fission as an energy source, and give rise to ongoing political debate over nuclear power.