I. Ch. 21.1 Nuclear Radiation
... What happens in a nuclear chain reaction? a) When the nuclei of certain isotopes are bombarded with neutrons, they undergo _________________, the splitting of a nucleus into smaller fragments. b) In a chain reaction, some of the neutrons produced react with other fissionable atoms, producing more ne ...
... What happens in a nuclear chain reaction? a) When the nuclei of certain isotopes are bombarded with neutrons, they undergo _________________, the splitting of a nucleus into smaller fragments. b) In a chain reaction, some of the neutrons produced react with other fissionable atoms, producing more ne ...
isotope - Aurora City Schools
... together • Acts over short distances…only on particle next to it • That’s why many more neutrons at higher atomic number…more strong nuclear force ...
... together • Acts over short distances…only on particle next to it • That’s why many more neutrons at higher atomic number…more strong nuclear force ...
Atomic Theory Handout CNS 8
... and the idea of "complementarity" -- that things may have a dual nature (as the electron is both particle and wave) but we can only experience one aspect at a time. ...
... and the idea of "complementarity" -- that things may have a dual nature (as the electron is both particle and wave) but we can only experience one aspect at a time. ...
Modern Physics TEST
... ____ 14. In fusion reactions, how does the binding energy per nucleon vary? a. The binding energy per nucleon remains constant as atomic number increases. b. The binding energy per nucleon remains constant as atomic number decreases. c. The binding energy per nucleon increases as atomic number incre ...
... ____ 14. In fusion reactions, how does the binding energy per nucleon vary? a. The binding energy per nucleon remains constant as atomic number increases. b. The binding energy per nucleon remains constant as atomic number decreases. c. The binding energy per nucleon increases as atomic number incre ...
Chapter 1 Learning Objective Summary
... Chemical reactions involve the gain, loss, or sharing of the outer electrons, whereas nuclear reactions involve changes to the composition of the nucleus. This means that alchemy is possible (though not economical!), because transmutation of one element into another can be accomplished via radioacti ...
... Chemical reactions involve the gain, loss, or sharing of the outer electrons, whereas nuclear reactions involve changes to the composition of the nucleus. This means that alchemy is possible (though not economical!), because transmutation of one element into another can be accomplished via radioacti ...
Nuclear Decay
... Fission is a reaction when the nucleus of an atom, having captured a neutron, splits into two or more nuclei, and in so doing, releases a significant amount of energy as well as more neutrons. These neutrons then go on to split more nuclei and a chain reaction takes place. ...
... Fission is a reaction when the nucleus of an atom, having captured a neutron, splits into two or more nuclei, and in so doing, releases a significant amount of energy as well as more neutrons. These neutrons then go on to split more nuclei and a chain reaction takes place. ...
Lesson 13: Nuclear Propulsion Basics
... • However, in solid fuel they can only travel a microscopic distance, so their energy becomes converted into heat. • The balance of the energy comes from gamma rays emitted during or immediately following the fission process and from the kinetic energy of the neutrons. – Some of the latter are immed ...
... • However, in solid fuel they can only travel a microscopic distance, so their energy becomes converted into heat. • The balance of the energy comes from gamma rays emitted during or immediately following the fission process and from the kinetic energy of the neutrons. – Some of the latter are immed ...
+ → ep no - University of Iowa Physics
... fusing deuterium with tritium nuclei to form helium and neutrons • To achieve this, the hydrogen must be heated to 100 million C using a fission bomb thermonuclear • Thermonuclear fusion is the energy source in a star ...
... fusing deuterium with tritium nuclei to form helium and neutrons • To achieve this, the hydrogen must be heated to 100 million C using a fission bomb thermonuclear • Thermonuclear fusion is the energy source in a star ...
120 min This paper - University of Southampton
... the projectile is of the order of the nuclear radius. The part of the wavefront that passes at a distance r from the centre of the nucleus and is scattered through an angle has a phase difference qr/h̄ relative to the part of the wavefront that passes though the centre, where q is the momentum chang ...
... the projectile is of the order of the nuclear radius. The part of the wavefront that passes at a distance r from the centre of the nucleus and is scattered through an angle has a phase difference qr/h̄ relative to the part of the wavefront that passes though the centre, where q is the momentum chang ...
nuclear fusion
... This led to the formation of H nuclei. • The H nuclei were pulled together by gravity into masses that would become the stars. The H nuclei fused into He nuclei, releasing enough energy that the star began to shine. • The fusion process continued for billions of years, releasing energy as heavier an ...
... This led to the formation of H nuclei. • The H nuclei were pulled together by gravity into masses that would become the stars. The H nuclei fused into He nuclei, releasing enough energy that the star began to shine. • The fusion process continued for billions of years, releasing energy as heavier an ...
1.6--NOTES--Detecting Radiation Nuclear Rxtns
... • because you can’t see or feel radiation (alpha or beta particles or gamma rays), you must use special instruments to detect them. • each of these tools detects the ions produced by the radioactive particles as they collide with matter, not the radiation itself. • cloud chamber = a small glass box ...
... • because you can’t see or feel radiation (alpha or beta particles or gamma rays), you must use special instruments to detect them. • each of these tools detects the ions produced by the radioactive particles as they collide with matter, not the radiation itself. • cloud chamber = a small glass box ...
1 The Nucleus Total number of nucleons: mass number Number of
... inhibitors of a protein called HMGA-CoA reductase, and they help to control cholesterol biosynthesis and limit cardiovascular ...
... inhibitors of a protein called HMGA-CoA reductase, and they help to control cholesterol biosynthesis and limit cardiovascular ...
Radioactivity - Mrs. Sjuts` Science Site
... ! Some rocks contain uranium, which has two radioactive isotopes with long half-‐lives, both decaying into isotopes of lead ! By comparing the uranium isotope and the daughter nuclei the number of half ...
... ! Some rocks contain uranium, which has two radioactive isotopes with long half-‐lives, both decaying into isotopes of lead ! By comparing the uranium isotope and the daughter nuclei the number of half ...
FYS 3520-Midterm2014
... c) Fission decay. Discuss why the fission decay problem differs from that of neutron and gamma decay. d) Fission to neutron competition. Why are 235U, 233U and 239Pu fissile when hit by thermal neutron, while 238U and 232Th are not? e) Draw by hand the dependence of the fission cross section vs ener ...
... c) Fission decay. Discuss why the fission decay problem differs from that of neutron and gamma decay. d) Fission to neutron competition. Why are 235U, 233U and 239Pu fissile when hit by thermal neutron, while 238U and 232Th are not? e) Draw by hand the dependence of the fission cross section vs ener ...
Power point
... Converting potential energy to emission of “prompt” neutrons Gamma emission after neutrons Then decay Occasionally one of these decays populates a high lying excited state of a daughter that is unstable with respect to neutron emission * “delayed” neutrons • Neutron spatial distribution ...
... Converting potential energy to emission of “prompt” neutrons Gamma emission after neutrons Then decay Occasionally one of these decays populates a high lying excited state of a daughter that is unstable with respect to neutron emission * “delayed” neutrons • Neutron spatial distribution ...
Document
... must contain the same number of protons. They may contain varying numbers of neutrons. Isotopes of an element have the same Z but differing N and A values. Example: 11 12 13 14 ...
... must contain the same number of protons. They may contain varying numbers of neutrons. Isotopes of an element have the same Z but differing N and A values. Example: 11 12 13 14 ...
Nuclear Fission and Fusion Notes
... and p attract other n and p. *n have no charge and they do not repel each other or the protons. *p repel each other with the electric force and attract each other with the strong nuclear force. ...
... and p attract other n and p. *n have no charge and they do not repel each other or the protons. *p repel each other with the electric force and attract each other with the strong nuclear force. ...
Foldable - Georgetown ISD
... Gamma (γ) Rays (*deadliest form of radiation) – no particle (only radiation/energy) Shielding: thick layers of lead or concrete Fission: *splitting an atom into smaller atoms (also releases energy), *a very heavy nucleus splits into more stable, intermediate-size nuclei Uses: nuclear power plants (* ...
... Gamma (γ) Rays (*deadliest form of radiation) – no particle (only radiation/energy) Shielding: thick layers of lead or concrete Fission: *splitting an atom into smaller atoms (also releases energy), *a very heavy nucleus splits into more stable, intermediate-size nuclei Uses: nuclear power plants (* ...
File
... Radioactivity: “the release of nuclear radiation in the form of particles & rays from a radioactive element.” Isotopes are often unstable – they have more neutrons than the element “wants” The isotopes are naturally occurring & decompose at different rates depending on the type of element. A ...
... Radioactivity: “the release of nuclear radiation in the form of particles & rays from a radioactive element.” Isotopes are often unstable – they have more neutrons than the element “wants” The isotopes are naturally occurring & decompose at different rates depending on the type of element. A ...
Nuclear Chemistry - Northwest ISD Moodle
... Mass number does not change Add 1 to atomic number Identify new element from atomic number Add beta particle ...
... Mass number does not change Add 1 to atomic number Identify new element from atomic number Add beta particle ...
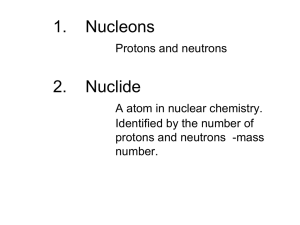
1. Nucleons Protons and neutrons 2. Nuclide A atom in
... type of radioactive decay called electron capture, an inner orbital electron is captured by the nucleus of its own atom. • Gamma rays- high energy emr waves emitted from a nucleus as it changes from ground state to excited state. ...
... type of radioactive decay called electron capture, an inner orbital electron is captured by the nucleus of its own atom. • Gamma rays- high energy emr waves emitted from a nucleus as it changes from ground state to excited state. ...
Nuclear Reactions
... The missing mass (mass defect) has been stored as energy in the nucleus. It is called the binding energy of the nucleus. ...
... The missing mass (mass defect) has been stored as energy in the nucleus. It is called the binding energy of the nucleus. ...
Nuclear - chemmybear.com
... and write the balanced nuclear reaction for that less. decay process. (c) The neutron/proton ratio in Sr-90 and Cs-137 is (c) Gamma rays are observed during the radioactive too large and they emit beta particles (converting decay of carbon-11. Why is it unnecessary to inneutrons into protons) to low ...
... and write the balanced nuclear reaction for that less. decay process. (c) The neutron/proton ratio in Sr-90 and Cs-137 is (c) Gamma rays are observed during the radioactive too large and they emit beta particles (converting decay of carbon-11. Why is it unnecessary to inneutrons into protons) to low ...
Nuclear fission
In nuclear physics and nuclear chemistry, nuclear fission is either a nuclear reaction or a radioactive decay process in which the nucleus of an atom splits into smaller parts (lighter nuclei). The fission process often produces free neutrons and photons (in the form of gamma rays), and releases a very large amount of energy even by the energetic standards of radioactive decay.Nuclear fission of heavy elements was discovered on December 17, 1938 by German Otto Hahn and his assistant Fritz Strassmann, and explained theoretically in January 1939 by Lise Meitner and her nephew Otto Robert Frisch. Frisch named the process by analogy with biological fission of living cells. It is an exothermic reaction which can release large amounts of energy both as electromagnetic radiation and as kinetic energy of the fragments (heating the bulk material where fission takes place). In order for fission to produce energy, the total binding energy of the resulting elements must be less negative (higher energy) than that of the starting element.Fission is a form of nuclear transmutation because the resulting fragments are not the same element as the original atom. The two nuclei produced are most often of comparable but slightly different sizes, typically with a mass ratio of products of about 3 to 2, for common fissile isotopes. Most fissions are binary fissions (producing two charged fragments), but occasionally (2 to 4 times per 1000 events), three positively charged fragments are produced, in a ternary fission. The smallest of these fragments in ternary processes ranges in size from a proton to an argon nucleus.Apart from fission induced by a neutron, harnessed and exploited by humans, a natural form of spontaneous radioactive decay (not requiring a neutron) is also referred to as fission, and occurs especially in very high-mass-number isotopes. Spontaneous fission was discovered in 1940 by Flyorov, Petrzhak and Kurchatov in Moscow, when they decided to confirm that, without bombardment by neutrons, the fission rate of uranium was indeed negligible, as predicted by Niels Bohr; it wasn't.The unpredictable composition of the products (which vary in a broad probabilistic and somewhat chaotic manner) distinguishes fission from purely quantum-tunnelling processes such as proton emission, alpha decay and cluster decay, which give the same products each time. Nuclear fission produces energy for nuclear power and drives the explosion of nuclear weapons. Both uses are possible because certain substances called nuclear fuels undergo fission when struck by fission neutrons, and in turn emit neutrons when they break apart. This makes possible a self-sustaining nuclear chain reaction that releases energy at a controlled rate in a nuclear reactor or at a very rapid uncontrolled rate in a nuclear weapon.The amount of free energy contained in nuclear fuel is millions of times the amount of free energy contained in a similar mass of chemical fuel such as gasoline, making nuclear fission a very dense source of energy. The products of nuclear fission, however, are on average far more radioactive than the heavy elements which are normally fissioned as fuel, and remain so for significant amounts of time, giving rise to a nuclear waste problem. Concerns over nuclear waste accumulation and over the destructive potential of nuclear weapons may counterbalance the desirable qualities of fission as an energy source, and give rise to ongoing political debate over nuclear power.