Chemistry Standard 2A-Nucleus Section 20.1
... b. atomic number d. neutron-to-proton ratio 5. What is the process in which an unstable atomic nucleus emits charged particles or energy or both? Page answer is found ____________ a. radioactivity c. decomposition b. oxidation d. none of the above 6. When the ____ is not large enough to hold a nucle ...
... b. atomic number d. neutron-to-proton ratio 5. What is the process in which an unstable atomic nucleus emits charged particles or energy or both? Page answer is found ____________ a. radioactivity c. decomposition b. oxidation d. none of the above 6. When the ____ is not large enough to hold a nucle ...
IB-ATOMIC-AND-NUCLEAR-PHYSICS-DEFINITIONS
... RADIOACTIVE DECAY: A random and spontaneous process in which unstable nuclei emit a particle (disintegrate). The rate decreases exponentially with time. NATURAL RADIOACTIVE DECAY: The emission of an alpha or beta particle. NUCLEAR STRONG FORCE: The force that holds the particles of a nucleus togethe ...
... RADIOACTIVE DECAY: A random and spontaneous process in which unstable nuclei emit a particle (disintegrate). The rate decreases exponentially with time. NATURAL RADIOACTIVE DECAY: The emission of an alpha or beta particle. NUCLEAR STRONG FORCE: The force that holds the particles of a nucleus togethe ...
Nuclear Power Plant Notes
... • The half-life of an isotope is the amount of time it takes for half of the atoms to decay into a more stable form. • Naturally abundant isotopes exist around us because their half-lives are longer than the age of the earth. ...
... • The half-life of an isotope is the amount of time it takes for half of the atoms to decay into a more stable form. • Naturally abundant isotopes exist around us because their half-lives are longer than the age of the earth. ...
Radioactivity - Williamstown Independent Schools
... materials that can be safely placed in the body to track movement of materials. • Radiation can also be used to kill cancerous ...
... materials that can be safely placed in the body to track movement of materials. • Radiation can also be used to kill cancerous ...
NUCLEAR CHEMISTRY PACKET - Student
... produce three different kinds of radiation. He separated the radiation according to their penetrating abilities and named them α (alpha), β (beta) and γ (gamma) after the first three letters in the Greek alphabet. ...
... produce three different kinds of radiation. He separated the radiation according to their penetrating abilities and named them α (alpha), β (beta) and γ (gamma) after the first three letters in the Greek alphabet. ...
Objectives for Nuclear Chemistry
... Fission reactions result in the splitting of heavier nuclei into lighter ones. This is accomplished by hitting the large nucleus with a slow moving neutron, the nucleus absorbs the neutron and becomes unstable. The new unstable nucleus then breaks apart into fission fragments of elements of lighter ...
... Fission reactions result in the splitting of heavier nuclei into lighter ones. This is accomplished by hitting the large nucleus with a slow moving neutron, the nucleus absorbs the neutron and becomes unstable. The new unstable nucleus then breaks apart into fission fragments of elements of lighter ...
Nuclear Fusion
... Fission- splitting of nuclei into smaller nuclei Radioactive Decay or radioactivity Reactions begin with unstable isotopes called radioisotopes that undergo change to become stable ...
... Fission- splitting of nuclei into smaller nuclei Radioactive Decay or radioactivity Reactions begin with unstable isotopes called radioisotopes that undergo change to become stable ...
Nuclear Chemistry - Mona Shores Blogs
... uncontrolled chain reactions • In order for a chain reaction to keep going, the nucleus that splits needs to produce one neutron that causes the fission of another nucleus – The material reacting uncontrolled needs to have a critical mass. – CRITICAL MASS: the smallest possible mass of a fissionable ...
... uncontrolled chain reactions • In order for a chain reaction to keep going, the nucleus that splits needs to produce one neutron that causes the fission of another nucleus – The material reacting uncontrolled needs to have a critical mass. – CRITICAL MASS: the smallest possible mass of a fissionable ...
Chem 1721 Brief Notes: Chapter 20 Chapter 20: Nuclear Chemistry
... note: isotope nomenclature involves naming the element and indicating the mass number i.e. calcium – 42 or calcium – 44 isotope symbols show the mass number superscripted in front of the element symbol; may or may not be combined with the atomic number subscripted in front of element symbol i.e. ...
... note: isotope nomenclature involves naming the element and indicating the mass number i.e. calcium – 42 or calcium – 44 isotope symbols show the mass number superscripted in front of the element symbol; may or may not be combined with the atomic number subscripted in front of element symbol i.e. ...
first lecture - الدكتورة / زينب بنت زكي الفل
... The nucleus of an atom is abound quantum system and hence can exist in different quantum states characterized by their energies ,angular momenta etc. the lowest energy state is known as as the ground state and the nuclei normally exist in this state , the properties of the nuclei which will be discu ...
... The nucleus of an atom is abound quantum system and hence can exist in different quantum states characterized by their energies ,angular momenta etc. the lowest energy state is known as as the ground state and the nuclei normally exist in this state , the properties of the nuclei which will be discu ...
Beryllium isotopes in geochronology Cosmogenic Be and Be
... fission – the spontaneous (or induced by particle collision) splitting of a heavy nucleus into a pair (only rarely more) of nearly equal fission fragments (fission products) generally with some neutrons. Fission is accompanied by the release of a large quantity of energy. [return] gamma rays (gamma ...
... fission – the spontaneous (or induced by particle collision) splitting of a heavy nucleus into a pair (only rarely more) of nearly equal fission fragments (fission products) generally with some neutrons. Fission is accompanied by the release of a large quantity of energy. [return] gamma rays (gamma ...
File - Chemistry with Mr. Patmos
... Have atomic numbers above 92 undergo transmutation Do not occur in nature Have been synthesized in nuclear reactors or particle accelerators Are radioactive ...
... Have atomic numbers above 92 undergo transmutation Do not occur in nature Have been synthesized in nuclear reactors or particle accelerators Are radioactive ...
Nuclear Chemistry
... characteristic of some elements.(radioisotopes). • While comparing the activity of pure uranium to a uranium ore sample, they found that the ore was significantly more radioactive than the pure material. They concluded that the ore contained additional radioactive components besides the uranium. Thi ...
... characteristic of some elements.(radioisotopes). • While comparing the activity of pure uranium to a uranium ore sample, they found that the ore was significantly more radioactive than the pure material. They concluded that the ore contained additional radioactive components besides the uranium. Thi ...
Nuclear power LEDE-HISTORY version C 1. Fermi thought he had
... a) less b) about the same c) greater ...
... a) less b) about the same c) greater ...
CHAPTER 13: Nuclear Interactions and Applications
... Fast breeder reactors have been built that convert 238U to 239Pu. The reactors are designed to use fast neutrons. Breeder reactors hold the promise of providing an almost unlimited supply of fissionable material. One of the downsides of such reactors is that plutonium is highly toxic, and there is c ...
... Fast breeder reactors have been built that convert 238U to 239Pu. The reactors are designed to use fast neutrons. Breeder reactors hold the promise of providing an almost unlimited supply of fissionable material. One of the downsides of such reactors is that plutonium is highly toxic, and there is c ...
Isotope Notes
... i. It is always the same for a given element and is always a whole number. ii. It can be found on the periodic table. b. The MASS NUMBER must be given in order for you to determine the number of neutrons. i. It is NOT on the periodic table. c. In a neutral atom, the number of electrons is equal to t ...
... i. It is always the same for a given element and is always a whole number. ii. It can be found on the periodic table. b. The MASS NUMBER must be given in order for you to determine the number of neutrons. i. It is NOT on the periodic table. c. In a neutral atom, the number of electrons is equal to t ...
NUCLEAR CHEMISTRY REVIEW SHEET
... _____ 8. In the nuclear decay process called electron capture, what change occurs to the atomic number of the capturing atom? a. It increases by one b. It decreases by one c. It increases by two d. It decreases by two _____ 9. In the nuclear decay process called electron capture, what change occurs ...
... _____ 8. In the nuclear decay process called electron capture, what change occurs to the atomic number of the capturing atom? a. It increases by one b. It decreases by one c. It increases by two d. It decreases by two _____ 9. In the nuclear decay process called electron capture, what change occurs ...
Radioactive Reactions
... Radioactive Reactions • When an atom emits part of its NUCLEUS (protons or neutrons) this is called radiation • This happens because the nucleus is unstable. • When an atom emits protons its identity changes • This can happen naturally (sun) or through man made isotopes in a lab ...
... Radioactive Reactions • When an atom emits part of its NUCLEUS (protons or neutrons) this is called radiation • This happens because the nucleus is unstable. • When an atom emits protons its identity changes • This can happen naturally (sun) or through man made isotopes in a lab ...
Nuclear chemistry – the study of nuclear reactions and their uses in
... Strong nuclear forces exist between nucleons. The belt of stability, where all stable nuclei are found, ends at element 83; anything with 84 or more protons are radioactive. Radioactive decay depends to a large extend on its neutron-to-proton ratio. i. Nuclei above the belt of stability (high neutro ...
... Strong nuclear forces exist between nucleons. The belt of stability, where all stable nuclei are found, ends at element 83; anything with 84 or more protons are radioactive. Radioactive decay depends to a large extend on its neutron-to-proton ratio. i. Nuclei above the belt of stability (high neutro ...
Nuclear Chemistry
... • Gamma rays are emitted during nuclear reactions, either alone or with other types of radiation • Gamma rays do NOT change the mass number or atomic number because they are energy not matter. ...
... • Gamma rays are emitted during nuclear reactions, either alone or with other types of radiation • Gamma rays do NOT change the mass number or atomic number because they are energy not matter. ...
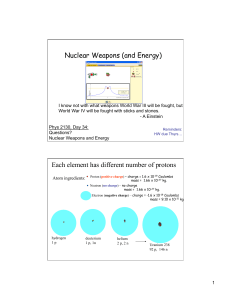
Nuclear Weapons (and Energy) Each element has different number
... produced the energy equivalent of 22 ktons of TNT = 8.8 x 1013 J. 17% of the plutonium atoms underwent fission. " How long would this power your house? How much power (energy / sec) do you use? ...
... produced the energy equivalent of 22 ktons of TNT = 8.8 x 1013 J. 17% of the plutonium atoms underwent fission. " How long would this power your house? How much power (energy / sec) do you use? ...
2005 Nuclear FRQs - AP Chemistry Olympics
... and write the balanced nuclear reaction for that less. decay process. (c) The neutron/proton ratio in Sr-90 and Cs-137 is (c) Gamma rays are observed during the radioactive too large and they emit beta particles (converting decay of carbon-11. Why is it unnecessary to inneutrons into protons) to low ...
... and write the balanced nuclear reaction for that less. decay process. (c) The neutron/proton ratio in Sr-90 and Cs-137 is (c) Gamma rays are observed during the radioactive too large and they emit beta particles (converting decay of carbon-11. Why is it unnecessary to inneutrons into protons) to low ...
radioactive decay - Aurora City Schools
... forces holds nucleus together • Acts over short distances…only on particle next to it • That’s why many more neutrons at higher atomic number…more strong nuclear force ...
... forces holds nucleus together • Acts over short distances…only on particle next to it • That’s why many more neutrons at higher atomic number…more strong nuclear force ...
Nuclear fission
In nuclear physics and nuclear chemistry, nuclear fission is either a nuclear reaction or a radioactive decay process in which the nucleus of an atom splits into smaller parts (lighter nuclei). The fission process often produces free neutrons and photons (in the form of gamma rays), and releases a very large amount of energy even by the energetic standards of radioactive decay.Nuclear fission of heavy elements was discovered on December 17, 1938 by German Otto Hahn and his assistant Fritz Strassmann, and explained theoretically in January 1939 by Lise Meitner and her nephew Otto Robert Frisch. Frisch named the process by analogy with biological fission of living cells. It is an exothermic reaction which can release large amounts of energy both as electromagnetic radiation and as kinetic energy of the fragments (heating the bulk material where fission takes place). In order for fission to produce energy, the total binding energy of the resulting elements must be less negative (higher energy) than that of the starting element.Fission is a form of nuclear transmutation because the resulting fragments are not the same element as the original atom. The two nuclei produced are most often of comparable but slightly different sizes, typically with a mass ratio of products of about 3 to 2, for common fissile isotopes. Most fissions are binary fissions (producing two charged fragments), but occasionally (2 to 4 times per 1000 events), three positively charged fragments are produced, in a ternary fission. The smallest of these fragments in ternary processes ranges in size from a proton to an argon nucleus.Apart from fission induced by a neutron, harnessed and exploited by humans, a natural form of spontaneous radioactive decay (not requiring a neutron) is also referred to as fission, and occurs especially in very high-mass-number isotopes. Spontaneous fission was discovered in 1940 by Flyorov, Petrzhak and Kurchatov in Moscow, when they decided to confirm that, without bombardment by neutrons, the fission rate of uranium was indeed negligible, as predicted by Niels Bohr; it wasn't.The unpredictable composition of the products (which vary in a broad probabilistic and somewhat chaotic manner) distinguishes fission from purely quantum-tunnelling processes such as proton emission, alpha decay and cluster decay, which give the same products each time. Nuclear fission produces energy for nuclear power and drives the explosion of nuclear weapons. Both uses are possible because certain substances called nuclear fuels undergo fission when struck by fission neutrons, and in turn emit neutrons when they break apart. This makes possible a self-sustaining nuclear chain reaction that releases energy at a controlled rate in a nuclear reactor or at a very rapid uncontrolled rate in a nuclear weapon.The amount of free energy contained in nuclear fuel is millions of times the amount of free energy contained in a similar mass of chemical fuel such as gasoline, making nuclear fission a very dense source of energy. The products of nuclear fission, however, are on average far more radioactive than the heavy elements which are normally fissioned as fuel, and remain so for significant amounts of time, giving rise to a nuclear waste problem. Concerns over nuclear waste accumulation and over the destructive potential of nuclear weapons may counterbalance the desirable qualities of fission as an energy source, and give rise to ongoing political debate over nuclear power.