nuclear chemistry - Magoffin County Schools
... including RADIOACTIVE DECAY, NUCLEAR FISSION and NUCLEAR FUSION. ...
... including RADIOACTIVE DECAY, NUCLEAR FISSION and NUCLEAR FUSION. ...
Ch 21 Nuclear - coolchemistrystuff
... -Example 2: List the following elements in greatest to least stability: sodium-24, helium-4, calcium-40 helium-4 (two magic numbers, both even) > calcium-40 (one magic number, both even) > sodium-24 III. Rates of Radioactive Decay Half-life: the time required for half of a sample of a particular r ...
... -Example 2: List the following elements in greatest to least stability: sodium-24, helium-4, calcium-40 helium-4 (two magic numbers, both even) > calcium-40 (one magic number, both even) > sodium-24 III. Rates of Radioactive Decay Half-life: the time required for half of a sample of a particular r ...
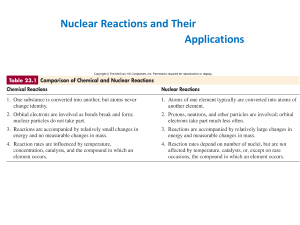
Nuclear Reactions and Their Applications
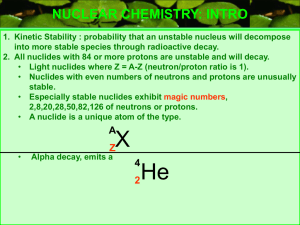
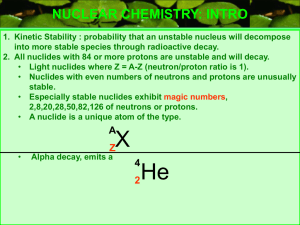
... Nuclides with 84 or more protons are unstable. Light nuclides are stable when Z equals A – Z (neutron/proton ratio is 1). For heavier elements the neutron/proton ratio required for stability is greater than 1 and increases with Z. Certain combinations of protons and neutrons seem to confer special s ...
... Nuclides with 84 or more protons are unstable. Light nuclides are stable when Z equals A – Z (neutron/proton ratio is 1). For heavier elements the neutron/proton ratio required for stability is greater than 1 and increases with Z. Certain combinations of protons and neutrons seem to confer special s ...
Name
... reactions 6. Chain reactions can be controlled a. If there is less than a critical mass of a fissionable isotope, a chain reaction will not occur 7. The critical mass is the minimum mass of a fissionable isotope that provides the number of neutrons needed to sustain a chain reaction D. Nuclear Fusio ...
... reactions 6. Chain reactions can be controlled a. If there is less than a critical mass of a fissionable isotope, a chain reaction will not occur 7. The critical mass is the minimum mass of a fissionable isotope that provides the number of neutrons needed to sustain a chain reaction D. Nuclear Fusio ...
NUCLEAR CHEMISTRY
... Shielding – radiation-absorbing material used to decrease the emission of radiation, especially gamma rays, from nuclear reactors. Control rods – neutron-absorbing rods that help control the reaction by limiting free neutrons. Moderator – used to slow down the fast neutrons produced by fission Urani ...
... Shielding – radiation-absorbing material used to decrease the emission of radiation, especially gamma rays, from nuclear reactors. Control rods – neutron-absorbing rods that help control the reaction by limiting free neutrons. Moderator – used to slow down the fast neutrons produced by fission Urani ...
NUCLEAR CHEMISTRY: INTRO
... NUCLEAR CHEMISTRY: INTRO 1. Kinetic Stability : probability that an unstable nucleus will decompose into more stable species through radioactive decay. 2. All nuclides with 84 or more protons are unstable and will decay. • Light nuclides where Z = A-Z (neutron/proton ratio is 1). • Nuclides with eve ...
... NUCLEAR CHEMISTRY: INTRO 1. Kinetic Stability : probability that an unstable nucleus will decompose into more stable species through radioactive decay. 2. All nuclides with 84 or more protons are unstable and will decay. • Light nuclides where Z = A-Z (neutron/proton ratio is 1). • Nuclides with eve ...
(neutron/proton ratio is 1).
... NUCLEAR CHEMISTRY: INTRO 1. Kinetic Stability : probability that an unstable nucleus will decompose into more stable species through radioactive decay. 2. All nuclides with 84 or more protons are unstable and will decay. • Light nuclides where Z = A-Z (neutron/proton ratio is 1). • Nuclides with eve ...
... NUCLEAR CHEMISTRY: INTRO 1. Kinetic Stability : probability that an unstable nucleus will decompose into more stable species through radioactive decay. 2. All nuclides with 84 or more protons are unstable and will decay. • Light nuclides where Z = A-Z (neutron/proton ratio is 1). • Nuclides with eve ...
Slide 1
... an n0 is actually about 5 x 10-4 u more massive an e- has a mass of about 5 x 10-4 u…hmmm ...
... an n0 is actually about 5 x 10-4 u more massive an e- has a mass of about 5 x 10-4 u…hmmm ...
CHM 123-Chapter 2.7
... The algebraic sum of the subscripts must be the same on both side of the equation, and the algebraic sum of the superscripts must be the same on both side of the equation. ...
... The algebraic sum of the subscripts must be the same on both side of the equation, and the algebraic sum of the superscripts must be the same on both side of the equation. ...
2.10 Basic Nuclear Chemistry
... 1. Hyphen notation – the name followed by the Atomic mass. For example, Radium-228 2. Nuclear notation – Atomic mass over Atomic Number followed by Symbol. For example, 22888 Ra D. Nuclear Force 1. Short-range attractive forces that help hold together the nucleus of an atom. A. Nuclear Binding Energ ...
... 1. Hyphen notation – the name followed by the Atomic mass. For example, Radium-228 2. Nuclear notation – Atomic mass over Atomic Number followed by Symbol. For example, 22888 Ra D. Nuclear Force 1. Short-range attractive forces that help hold together the nucleus of an atom. A. Nuclear Binding Energ ...
Nuclear Chemistry
... A Beta particle is an electron created and emitted when a neutron is transformed* into a proton and an electron during radioactive decay. This action adds a proton and thus changes the identity of the atom. The mass number stays the same. ...
... A Beta particle is an electron created and emitted when a neutron is transformed* into a proton and an electron during radioactive decay. This action adds a proton and thus changes the identity of the atom. The mass number stays the same. ...
NUCLEAR CHEMISTRY
... normally strong enough to hold the protons and neutrons together. However, sometimes the force of repulsion due to the protons having the same charge overcomes the strong nuclear force and the atom breaks apart. ...
... normally strong enough to hold the protons and neutrons together. However, sometimes the force of repulsion due to the protons having the same charge overcomes the strong nuclear force and the atom breaks apart. ...
Radioactivity - Science 9
... An element that gives off nuclear radiation by releasing particles and rays is said to be radioactive. Radioactivity cannot be seen. However, it can be detected because it: can alter photographic film produces an electric charge in the surrounding air can produce fluorescence (glowing) Nucle ...
... An element that gives off nuclear radiation by releasing particles and rays is said to be radioactive. Radioactivity cannot be seen. However, it can be detected because it: can alter photographic film produces an electric charge in the surrounding air can produce fluorescence (glowing) Nucle ...
Nuclear Chemistry - Mrs. Carlyle`s Classroom
... Half-lives can be short as a fraction of a second or as long as several million years. Artificial radioisotopes have very short half-lives. Good for nuclear medicine. It is possible to use this method to date rocks as old as our solar system. ...
... Half-lives can be short as a fraction of a second or as long as several million years. Artificial radioisotopes have very short half-lives. Good for nuclear medicine. It is possible to use this method to date rocks as old as our solar system. ...
6.3 Nuclear Reactions
... after binding may be fractionally small. • For systems with high binding energies, however, the missing mass may be an easily measurable fraction. ...
... after binding may be fractionally small. • For systems with high binding energies, however, the missing mass may be an easily measurable fraction. ...
30.1 Radioactivity The atom is the smallest unit of achemical
... There are two types of the beta decay. The one is the βdecay and the other is the β+ decay. a- β• neutron decays into a proton • it has the same charge as electron • it has the same mass as electron • it can penetrate with few meters in air. 2 or 3 cm of wood are enough to protect oneself. ...
... There are two types of the beta decay. The one is the βdecay and the other is the β+ decay. a- β• neutron decays into a proton • it has the same charge as electron • it has the same mass as electron • it can penetrate with few meters in air. 2 or 3 cm of wood are enough to protect oneself. ...
Chemistry Test: Transmutation Multiple Choice 1. Identify the new
... Identify the new element when an alpha particle is emitted from Pu-244. a. Cm-240 b. Am-244 c. U-240 d. Np-244 Which of the following lists ranks nuclear radiation from most massive to least massive? a. alpha, beta, and gamma c. gamma, alpha, and beta b. beta, gamma, and alpha d. gamma, beta, and al ...
... Identify the new element when an alpha particle is emitted from Pu-244. a. Cm-240 b. Am-244 c. U-240 d. Np-244 Which of the following lists ranks nuclear radiation from most massive to least massive? a. alpha, beta, and gamma c. gamma, alpha, and beta b. beta, gamma, and alpha d. gamma, beta, and al ...
Quanta to Quarks part 2 - Connecting-Sharing-and
... two lighter nuclei, each of which is more stable than the original nucleus. The first artificially induced nuclear fission reaction was achieved by Enrico Fermi in 1934, although at the time he did not realise that fission had occurred. Fermi bombarded uranium with neutrons and produced radioactive ...
... two lighter nuclei, each of which is more stable than the original nucleus. The first artificially induced nuclear fission reaction was achieved by Enrico Fermi in 1934, although at the time he did not realise that fission had occurred. Fermi bombarded uranium with neutrons and produced radioactive ...
GLOSSARY OF SCIENTIFIC TERMS IN THE MYSTERY OF MATTER
... A unit that measures the effect of ionizing radiation upon a particular person. A group of two or more atoms linked together by sharing electrons in a chemical bond. A heavy, neutral particle in an atom’s nucleus that accounts for almost all of each atom’s mass, in addition to protons. Any of the si ...
... A unit that measures the effect of ionizing radiation upon a particular person. A group of two or more atoms linked together by sharing electrons in a chemical bond. A heavy, neutral particle in an atom’s nucleus that accounts for almost all of each atom’s mass, in addition to protons. Any of the si ...
E = mc2 (Einstein)
... The energy released by radioactive isotopes takes different forms. Society is primarily interested in the heat released, as we shall see in Chapters 13 and 14. However, some alpha, beta and gamma radiation inevitably accompanies this release of heat. We can view this radiation as “nuclear pollution, ...
... The energy released by radioactive isotopes takes different forms. Society is primarily interested in the heat released, as we shall see in Chapters 13 and 14. However, some alpha, beta and gamma radiation inevitably accompanies this release of heat. We can view this radiation as “nuclear pollution, ...
Concept Review 3.1 Introduction to Nuclear
... Electrostatic Force – Like charges repel (+, + and -, -) and opposite charges attract (-, +). As the number of protons in a nucleus increases, the repulsive electrostatic force between protons increases faster than the nuclear force. More neutrons are required to increase the nuclear force and sta ...
... Electrostatic Force – Like charges repel (+, + and -, -) and opposite charges attract (-, +). As the number of protons in a nucleus increases, the repulsive electrostatic force between protons increases faster than the nuclear force. More neutrons are required to increase the nuclear force and sta ...
Chapter 30: Nuclear Physics What will we learn in this chapter?
... Nuclear fission Definition: ! Decay process in which an unstable nucleus splits into two ! fragments of comparable mass (and often some neutrons), ! instead of emitting alpha or beta particles. Properties: The energy released in the fission is enormous (about 200MeV per nucleus) and appears as kine ...
... Nuclear fission Definition: ! Decay process in which an unstable nucleus splits into two ! fragments of comparable mass (and often some neutrons), ! instead of emitting alpha or beta particles. Properties: The energy released in the fission is enormous (about 200MeV per nucleus) and appears as kine ...
File
... UNIT 4 Periodicity & Nuclear Chemistry Common Assessment 16. In the figure below, what type of nuclear activity is represented? ...
... UNIT 4 Periodicity & Nuclear Chemistry Common Assessment 16. In the figure below, what type of nuclear activity is represented? ...
25.3 Fission and Fusion of Atomic Nuclei
... The sun, directly and indirectly, is the source of most energy used on Earth. The energy released by the sun results from nuclear fusion. Fusion occurs when nuclei combine to produce a nucleus of greater mass. In solar fusion, hydrogen nuclei (protons) fuse to make helium nuclei. Figure 25.13 shows ...
... The sun, directly and indirectly, is the source of most energy used on Earth. The energy released by the sun results from nuclear fusion. Fusion occurs when nuclei combine to produce a nucleus of greater mass. In solar fusion, hydrogen nuclei (protons) fuse to make helium nuclei. Figure 25.13 shows ...
Nuclear fission
In nuclear physics and nuclear chemistry, nuclear fission is either a nuclear reaction or a radioactive decay process in which the nucleus of an atom splits into smaller parts (lighter nuclei). The fission process often produces free neutrons and photons (in the form of gamma rays), and releases a very large amount of energy even by the energetic standards of radioactive decay.Nuclear fission of heavy elements was discovered on December 17, 1938 by German Otto Hahn and his assistant Fritz Strassmann, and explained theoretically in January 1939 by Lise Meitner and her nephew Otto Robert Frisch. Frisch named the process by analogy with biological fission of living cells. It is an exothermic reaction which can release large amounts of energy both as electromagnetic radiation and as kinetic energy of the fragments (heating the bulk material where fission takes place). In order for fission to produce energy, the total binding energy of the resulting elements must be less negative (higher energy) than that of the starting element.Fission is a form of nuclear transmutation because the resulting fragments are not the same element as the original atom. The two nuclei produced are most often of comparable but slightly different sizes, typically with a mass ratio of products of about 3 to 2, for common fissile isotopes. Most fissions are binary fissions (producing two charged fragments), but occasionally (2 to 4 times per 1000 events), three positively charged fragments are produced, in a ternary fission. The smallest of these fragments in ternary processes ranges in size from a proton to an argon nucleus.Apart from fission induced by a neutron, harnessed and exploited by humans, a natural form of spontaneous radioactive decay (not requiring a neutron) is also referred to as fission, and occurs especially in very high-mass-number isotopes. Spontaneous fission was discovered in 1940 by Flyorov, Petrzhak and Kurchatov in Moscow, when they decided to confirm that, without bombardment by neutrons, the fission rate of uranium was indeed negligible, as predicted by Niels Bohr; it wasn't.The unpredictable composition of the products (which vary in a broad probabilistic and somewhat chaotic manner) distinguishes fission from purely quantum-tunnelling processes such as proton emission, alpha decay and cluster decay, which give the same products each time. Nuclear fission produces energy for nuclear power and drives the explosion of nuclear weapons. Both uses are possible because certain substances called nuclear fuels undergo fission when struck by fission neutrons, and in turn emit neutrons when they break apart. This makes possible a self-sustaining nuclear chain reaction that releases energy at a controlled rate in a nuclear reactor or at a very rapid uncontrolled rate in a nuclear weapon.The amount of free energy contained in nuclear fuel is millions of times the amount of free energy contained in a similar mass of chemical fuel such as gasoline, making nuclear fission a very dense source of energy. The products of nuclear fission, however, are on average far more radioactive than the heavy elements which are normally fissioned as fuel, and remain so for significant amounts of time, giving rise to a nuclear waste problem. Concerns over nuclear waste accumulation and over the destructive potential of nuclear weapons may counterbalance the desirable qualities of fission as an energy source, and give rise to ongoing political debate over nuclear power.