Modern Physics - hrsbstaff.ednet.ns.ca
... Isotopes of a given element correspond to nuclei with different numbers of neutrons. This results in a variety of different properties for the nuclei, including the obvious one of mass. The chemical behavior, however, is governed by the lectrons. All isotopes of a given element have the same number ...
... Isotopes of a given element correspond to nuclei with different numbers of neutrons. This results in a variety of different properties for the nuclei, including the obvious one of mass. The chemical behavior, however, is governed by the lectrons. All isotopes of a given element have the same number ...
Nuclear Chemistry I: Radioactivity Reading: Moore chapter 20
... Radioactivity is defined as the spontaneous emission of energy and/or subatomic particles by unstable atomic nuclei. It may refer to the energy or particles emitted. Note: The basic building blocks of the nucleus, neutrons and protons, are referred to as nucleons; forms of an element with the same a ...
... Radioactivity is defined as the spontaneous emission of energy and/or subatomic particles by unstable atomic nuclei. It may refer to the energy or particles emitted. Note: The basic building blocks of the nucleus, neutrons and protons, are referred to as nucleons; forms of an element with the same a ...
Examination 1
... a breaking of bonds within the molecules that were reaction to form new bonds and new molecules. The identity of the atoms participating in the reaction remained the same - so in balancing the reaction we always made sure that the number of atoms of each element on both sides of the eqn. was the sam ...
... a breaking of bonds within the molecules that were reaction to form new bonds and new molecules. The identity of the atoms participating in the reaction remained the same - so in balancing the reaction we always made sure that the number of atoms of each element on both sides of the eqn. was the sam ...
Nuclear Chemistry
... a breaking of bonds within the molecules that were reaction to form new bonds and new molecules. The identity of the atoms participating in the reaction remained the same - so in balancing the reaction we always made sure that the number of atoms of each element on both sides of the eqn. was the sam ...
... a breaking of bonds within the molecules that were reaction to form new bonds and new molecules. The identity of the atoms participating in the reaction remained the same - so in balancing the reaction we always made sure that the number of atoms of each element on both sides of the eqn. was the sam ...
ppt-nuclear - SandersScienceStuff
... Fission • Fission means to break apart. Nuclear fission occurs when a nucleus splits apart into different fragments. • This generally occurs with atoms that have a mass number heavier than 60. • The nuclei do not always split the same way. Scientists have found 200 different products from the fissi ...
... Fission • Fission means to break apart. Nuclear fission occurs when a nucleus splits apart into different fragments. • This generally occurs with atoms that have a mass number heavier than 60. • The nuclei do not always split the same way. Scientists have found 200 different products from the fissi ...
1 0 +1 0 - davis.k12.ut.us
... 9. There are some elements within the periodic table that do not occur in nature, can you list a few? Technetium, neptunium, elements 93 and up… 10. How can synthetic elements be produced? Bombarding an atom with a neutron or alpha particle. 11. A 5.0 g sample of Lead-210 decays to approximately .6 ...
... 9. There are some elements within the periodic table that do not occur in nature, can you list a few? Technetium, neptunium, elements 93 and up… 10. How can synthetic elements be produced? Bombarding an atom with a neutron or alpha particle. 11. A 5.0 g sample of Lead-210 decays to approximately .6 ...
Chapter 32 Applied Nucleonics
... possibility of tapping the energy of the nucleus. Recall that it is a conversion of some of the nuclear binding energy into kinetic energy that characterizes both fission and fusion. The basis of this conversion can be seen from the binding energy per nucleon curve which was shown in Figure 31.1. It ...
... possibility of tapping the energy of the nucleus. Recall that it is a conversion of some of the nuclear binding energy into kinetic energy that characterizes both fission and fusion. The basis of this conversion can be seen from the binding energy per nucleon curve which was shown in Figure 31.1. It ...
Nuclear Chemistry powerpoint
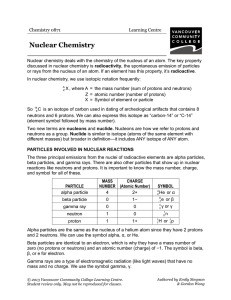
... ) and no charge ( ). Thus, it causes change in or numbers. Gamma rays almost accompany alpha and beta radiation. However, since there is effect on mass number or atomic number, they are usually from nuclear equations. ...
... ) and no charge ( ). Thus, it causes change in or numbers. Gamma rays almost accompany alpha and beta radiation. However, since there is effect on mass number or atomic number, they are usually from nuclear equations. ...
Nuclear Chemistry powerpoint
... ) and no charge ( ). Thus, it causes change in or numbers. Gamma rays almost accompany alpha and beta radiation. However, since there is effect on mass number or atomic number, they are usually from nuclear equations. ...
... ) and no charge ( ). Thus, it causes change in or numbers. Gamma rays almost accompany alpha and beta radiation. However, since there is effect on mass number or atomic number, they are usually from nuclear equations. ...
Nuclear Chemistry powerpoint
... ) and no charge ( ). Thus, it causes change in or numbers. Gamma rays almost accompany alpha and beta radiation. However, since there is effect on mass number or atomic number, they are usually from nuclear equations. ...
... ) and no charge ( ). Thus, it causes change in or numbers. Gamma rays almost accompany alpha and beta radiation. However, since there is effect on mass number or atomic number, they are usually from nuclear equations. ...
Masses in Atomic Units - proton 1.007 u 938.28 MeV
... - the forces binding protons and neutrons in the nucleus are much stronger (binding energy of MeV) than the forces binding electrons to the atom (binding energy of eV) - the constituents of a nucleus are called nucleons - a nucleus is characterized by its atomic number Z (the number of protons) and ...
... - the forces binding protons and neutrons in the nucleus are much stronger (binding energy of MeV) than the forces binding electrons to the atom (binding energy of eV) - the constituents of a nucleus are called nucleons - a nucleus is characterized by its atomic number Z (the number of protons) and ...
A Z N Atomic Mass: A = Z + N Mass and Rest Energy m =
... because the nuclear force is a much stronger force than that of the electric force caused by repulsion. The nuclear force is sometimes called the strong force. It is the interaction that binds nucleons together in the nucleus. The force works with dimensions on the nuclear level that are MUCH smalle ...
... because the nuclear force is a much stronger force than that of the electric force caused by repulsion. The nuclear force is sometimes called the strong force. It is the interaction that binds nucleons together in the nucleus. The force works with dimensions on the nuclear level that are MUCH smalle ...
6.2 Atomic Nucleus Stability and Isotopes
... Lighter atomic nuclei can fuse if high temperatures force them within a distance of a radius, allowing the strong nuclear force to bind them together. Heavier atomic nuclei can fission (split) if a particle is added that reduces the binding energy per nucleon and/or increases the repulsive force. Fi ...
... Lighter atomic nuclei can fuse if high temperatures force them within a distance of a radius, allowing the strong nuclear force to bind them together. Heavier atomic nuclei can fission (split) if a particle is added that reduces the binding energy per nucleon and/or increases the repulsive force. Fi ...
Unit 5 EW Tasks (1)
... ...the coolant should have a high specific heat heat... capacity. The role of control rods is described as to absorb ...movement of the rods varies the number of ...inserting the rods further into the reactor neutrons... neutrons absorbed... absorbs more neutrons (accept the converse). A constant po ...
... ...the coolant should have a high specific heat heat... capacity. The role of control rods is described as to absorb ...movement of the rods varies the number of ...inserting the rods further into the reactor neutrons... neutrons absorbed... absorbs more neutrons (accept the converse). A constant po ...
Name Period Nuclear Study Packet Set 1 1. What subatomic
... sample contains 34.2 mg of K-42. How much did it contain yesterday at the same time. 4. What percent of a sample of a radioactive element whose half life is 5 years will decay after 25 years? 5. What are some ways that nuclear reactions are being used today? Do not list any ways that we have tal ...
... sample contains 34.2 mg of K-42. How much did it contain yesterday at the same time. 4. What percent of a sample of a radioactive element whose half life is 5 years will decay after 25 years? 5. What are some ways that nuclear reactions are being used today? Do not list any ways that we have tal ...
Nuclear Chemistry powerpoint
... ) and no charge ( ). Thus, it causes change in or numbers. Gamma rays almost accompany alpha and beta radiation. However, since there is effect on mass number or atomic number, they are usually from nuclear equations. ...
... ) and no charge ( ). Thus, it causes change in or numbers. Gamma rays almost accompany alpha and beta radiation. However, since there is effect on mass number or atomic number, they are usually from nuclear equations. ...
Nuclear Chemistry powerpoint
... ) and no charge ( ). Thus, it causes change in or numbers. Gamma rays almost accompany alpha and beta radiation. However, since there is effect on mass number or atomic number, they are usually from nuclear equations. ...
... ) and no charge ( ). Thus, it causes change in or numbers. Gamma rays almost accompany alpha and beta radiation. However, since there is effect on mass number or atomic number, they are usually from nuclear equations. ...
Nuclear Chemistry powerpoint
... decay – alpha and beta particles and gamma ray emission Nuclear - emission of a or ...
... decay – alpha and beta particles and gamma ray emission Nuclear - emission of a or ...
Nuclear Chemistry - VCC Library
... neutrons and 6 protons. We can also express this isotope as “carbon-14” or “C-14” (element symbol followed by mass number). Two new terms are nucleons and nuclide. Nucleons are how we refer to protons and neutrons as a group. Nuclide is similar to isotope (atoms of the same element with different ma ...
... neutrons and 6 protons. We can also express this isotope as “carbon-14” or “C-14” (element symbol followed by mass number). Two new terms are nucleons and nuclide. Nucleons are how we refer to protons and neutrons as a group. Nuclide is similar to isotope (atoms of the same element with different ma ...
Nuclear reactions: fission and fusion
... The stray neutrons released by a spontaneous fission can prematurely initiate a chain reaction. This means that the assembly time to reach a critical mass has to be less than the rate of spontaneous fission. Scientists have to consider the spontaneous fission rate of each material when designing nuc ...
... The stray neutrons released by a spontaneous fission can prematurely initiate a chain reaction. This means that the assembly time to reach a critical mass has to be less than the rate of spontaneous fission. Scientists have to consider the spontaneous fission rate of each material when designing nuc ...
Nuclear Chemistry PowerPoint presentation
... Shielding – radiation-absorbing material used to decrease the emission of radiation, especially gamma rays, from nuclear reactors. Control rods – neutron-absorbing rods that help control the reaction by limiting free neutrons. Moderator – used to slow down the fast neutrons produced by fission Urani ...
... Shielding – radiation-absorbing material used to decrease the emission of radiation, especially gamma rays, from nuclear reactors. Control rods – neutron-absorbing rods that help control the reaction by limiting free neutrons. Moderator – used to slow down the fast neutrons produced by fission Urani ...
Chapter 25 Nuclear Chemistry
... All nuclei contain protons and neutrons (exception - hydrogen-1 has no neutrons). Since protons are positively charged, it would be expected that they would repel and separate, but this does not occur. A force holds them together. The nuclear force is an attractive force that acts between all nuclea ...
... All nuclei contain protons and neutrons (exception - hydrogen-1 has no neutrons). Since protons are positively charged, it would be expected that they would repel and separate, but this does not occur. A force holds them together. The nuclear force is an attractive force that acts between all nuclea ...
Nuclear fission
In nuclear physics and nuclear chemistry, nuclear fission is either a nuclear reaction or a radioactive decay process in which the nucleus of an atom splits into smaller parts (lighter nuclei). The fission process often produces free neutrons and photons (in the form of gamma rays), and releases a very large amount of energy even by the energetic standards of radioactive decay.Nuclear fission of heavy elements was discovered on December 17, 1938 by German Otto Hahn and his assistant Fritz Strassmann, and explained theoretically in January 1939 by Lise Meitner and her nephew Otto Robert Frisch. Frisch named the process by analogy with biological fission of living cells. It is an exothermic reaction which can release large amounts of energy both as electromagnetic radiation and as kinetic energy of the fragments (heating the bulk material where fission takes place). In order for fission to produce energy, the total binding energy of the resulting elements must be less negative (higher energy) than that of the starting element.Fission is a form of nuclear transmutation because the resulting fragments are not the same element as the original atom. The two nuclei produced are most often of comparable but slightly different sizes, typically with a mass ratio of products of about 3 to 2, for common fissile isotopes. Most fissions are binary fissions (producing two charged fragments), but occasionally (2 to 4 times per 1000 events), three positively charged fragments are produced, in a ternary fission. The smallest of these fragments in ternary processes ranges in size from a proton to an argon nucleus.Apart from fission induced by a neutron, harnessed and exploited by humans, a natural form of spontaneous radioactive decay (not requiring a neutron) is also referred to as fission, and occurs especially in very high-mass-number isotopes. Spontaneous fission was discovered in 1940 by Flyorov, Petrzhak and Kurchatov in Moscow, when they decided to confirm that, without bombardment by neutrons, the fission rate of uranium was indeed negligible, as predicted by Niels Bohr; it wasn't.The unpredictable composition of the products (which vary in a broad probabilistic and somewhat chaotic manner) distinguishes fission from purely quantum-tunnelling processes such as proton emission, alpha decay and cluster decay, which give the same products each time. Nuclear fission produces energy for nuclear power and drives the explosion of nuclear weapons. Both uses are possible because certain substances called nuclear fuels undergo fission when struck by fission neutrons, and in turn emit neutrons when they break apart. This makes possible a self-sustaining nuclear chain reaction that releases energy at a controlled rate in a nuclear reactor or at a very rapid uncontrolled rate in a nuclear weapon.The amount of free energy contained in nuclear fuel is millions of times the amount of free energy contained in a similar mass of chemical fuel such as gasoline, making nuclear fission a very dense source of energy. The products of nuclear fission, however, are on average far more radioactive than the heavy elements which are normally fissioned as fuel, and remain so for significant amounts of time, giving rise to a nuclear waste problem. Concerns over nuclear waste accumulation and over the destructive potential of nuclear weapons may counterbalance the desirable qualities of fission as an energy source, and give rise to ongoing political debate over nuclear power.