Word file
... identification of M23C6 carbides and MX carbonitrides, respectively, in the 0.078%C steels after heat treatment. The M23C6 carbides were identified as face-centered cubic structure having a lattice parameter of 1.067 nm. This is consistent with a reported result 1. The orientation relationship betwe ...
... identification of M23C6 carbides and MX carbonitrides, respectively, in the 0.078%C steels after heat treatment. The M23C6 carbides were identified as face-centered cubic structure having a lattice parameter of 1.067 nm. This is consistent with a reported result 1. The orientation relationship betwe ...
Conserving Matter - Hobbs High School
... ___ silver atoms ___ hydrogen atoms ___ sulfur atoms ___ oxygen atoms ...
... ___ silver atoms ___ hydrogen atoms ___ sulfur atoms ___ oxygen atoms ...
Vocabulary CHEM121
... Note the heavy stair-step line drawn between elements B & Al, etc. This line separates the metals (lower left) from the non-metals (upper right). Metals can only form cations. Non-metals form anions when combined with metals. Elements that touch the line are called metalloids (except Al, which ...
... Note the heavy stair-step line drawn between elements B & Al, etc. This line separates the metals (lower left) from the non-metals (upper right). Metals can only form cations. Non-metals form anions when combined with metals. Elements that touch the line are called metalloids (except Al, which ...
Scientific Method - Virtual Medical Academy
... The composition is uniform (WATER + SALT) HETEROGENEOUS: The composition is not uniform (WATER + SAND) Separation of Mixtures:-* Filtration . * distillation. * chromatography. ...
... The composition is uniform (WATER + SALT) HETEROGENEOUS: The composition is not uniform (WATER + SAND) Separation of Mixtures:-* Filtration . * distillation. * chromatography. ...
Materials on an Atomic Level
... an atom should have been. This is called a vacancy. It is also possible that there is an atom in a place where normally there would not be an atom. This event, which occurs in only very small concentrations, is called a self-interstitial. Naturally, materials consisting of only one element are impos ...
... an atom should have been. This is called a vacancy. It is also possible that there is an atom in a place where normally there would not be an atom. This event, which occurs in only very small concentrations, is called a self-interstitial. Naturally, materials consisting of only one element are impos ...
Power Point Slides P..
... Therefore are in higher energy state Surface energy, g [=] J/m2 Materials always try to reduce surface energy – tendency towards spherical shapes ...
... Therefore are in higher energy state Surface energy, g [=] J/m2 Materials always try to reduce surface energy – tendency towards spherical shapes ...
Scientific Method - Virtual Medical Academy
... The composition is uniform (WATER + SALT) HETEROGENEOUS: The composition is not uniform (WATER + SAND) Separation of Mixtures:-* Filtration . * distillation. * chromatography. ...
... The composition is uniform (WATER + SALT) HETEROGENEOUS: The composition is not uniform (WATER + SAND) Separation of Mixtures:-* Filtration . * distillation. * chromatography. ...
application of shape memory alloys in civil engineering
... The first field implementation using shape memory effect for posttensioning of a concrete structure was presented in (SOROUSHIAN, OSTOWARI et al. 2001). The shear cracks in the web of the reinforced concrete T-beam had an average width of 0.55 mm. For strengthening of the bridge girder, a harp-like ...
... The first field implementation using shape memory effect for posttensioning of a concrete structure was presented in (SOROUSHIAN, OSTOWARI et al. 2001). The shear cracks in the web of the reinforced concrete T-beam had an average width of 0.55 mm. For strengthening of the bridge girder, a harp-like ...
міністерство освіти і науки україни
... their molecules are arranged in a random manner some what as in the liquid state. For example, glass is commonly made from silicon dioxide or quartz sand, which has a crystalline structure. When the sand is melted and the liquid is cooled rapidly enough to avoid crystallization, an amorphous solid c ...
... their molecules are arranged in a random manner some what as in the liquid state. For example, glass is commonly made from silicon dioxide or quartz sand, which has a crystalline structure. When the sand is melted and the liquid is cooled rapidly enough to avoid crystallization, an amorphous solid c ...
Module 4 Trivia Review
... A Semi Conductor is a fancy name for a Metalloid. Semi means half or partial. So semiconductors (metalloids) have electrical conductivity half way between those of a conductor and an insulator (non-metal). Since they are solid and ductile, these metalloids have been found to be indispensable to the ...
... A Semi Conductor is a fancy name for a Metalloid. Semi means half or partial. So semiconductors (metalloids) have electrical conductivity half way between those of a conductor and an insulator (non-metal). Since they are solid and ductile, these metalloids have been found to be indispensable to the ...
x - How to make your homepage available
... Figure 9.3 (a) Pressuretemperature diagram for H2O. The triple point temperature is 273.0098 K and the triple point pressure is 4.6 torr. Notice the solid-liquid line sloping to the left. At normal pressure (1 atm or 760 torr), the melting temperature is 273 K. A possible scheme for freeze drying is ...
... Figure 9.3 (a) Pressuretemperature diagram for H2O. The triple point temperature is 273.0098 K and the triple point pressure is 4.6 torr. Notice the solid-liquid line sloping to the left. At normal pressure (1 atm or 760 torr), the melting temperature is 273 K. A possible scheme for freeze drying is ...
The Periodic Table - Harlan Independent Schools
... find the radioactive radium (Ra). While radium is not found around your house anymore, it used to be used in glow-in-thedark paints. The other elements are found in many items including fireworks, batteries, flashbulbs, and special alloys. The lighter alkaline earth metals such as magnesium and calc ...
... find the radioactive radium (Ra). While radium is not found around your house anymore, it used to be used in glow-in-thedark paints. The other elements are found in many items including fireworks, batteries, flashbulbs, and special alloys. The lighter alkaline earth metals such as magnesium and calc ...
CHEMISTRY SEMESTER ONE LAB 1 Lab 1: Stoichiometry and
... 3. Measure 30 mL of 1.0 M CuSO4 solution into a graduated cylinder. Pour it into an erlenmeyer flask, and heat gently to almost boiling. 4. Slowly add the hot CuSO4 solution to the beaker containing the iron powder. 5. Swirl the flask to insure the reaction goes to completion. When the reaction is c ...
... 3. Measure 30 mL of 1.0 M CuSO4 solution into a graduated cylinder. Pour it into an erlenmeyer flask, and heat gently to almost boiling. 4. Slowly add the hot CuSO4 solution to the beaker containing the iron powder. 5. Swirl the flask to insure the reaction goes to completion. When the reaction is c ...
Mini- Implant Materials: An Overview
... alloys, the presence of minimal amounts of interstitial elements such as hydrogen, nitrogen, oxygen and carbon can affect the mechanical properties in much more distinct manner. Titanium produces detectable embrittlement due to precipitation of titanium hydride when the alloy is slow cooled in alpha ...
... alloys, the presence of minimal amounts of interstitial elements such as hydrogen, nitrogen, oxygen and carbon can affect the mechanical properties in much more distinct manner. Titanium produces detectable embrittlement due to precipitation of titanium hydride when the alloy is slow cooled in alpha ...
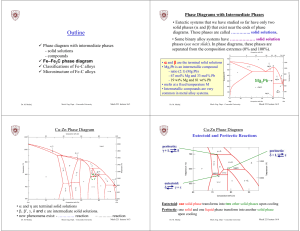
Outline - Concordia University
... • Microstructure depends on composition (carbon content) and heat treatment. • In the discussion below we consider slow cooling in which equilibrium is maintained. ...
... • Microstructure depends on composition (carbon content) and heat treatment. • In the discussion below we consider slow cooling in which equilibrium is maintained. ...
LATENT HEAT AND ELECTRODE POTENTIAL
... described the influence of temperature and of the state of the metallic electrodes on the electromotive force (emf) of a voltaic cell (1). In the copper-zinc Daniell cell he had found that sheet, electrodeposited, and other types of copper, combined with various types of zinc, produced essentially t ...
... described the influence of temperature and of the state of the metallic electrodes on the electromotive force (emf) of a voltaic cell (1). In the copper-zinc Daniell cell he had found that sheet, electrodeposited, and other types of copper, combined with various types of zinc, produced essentially t ...
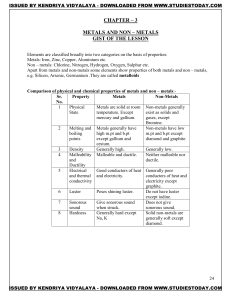
METALS AND NON – METALS Concepts
... 3. Solubility: soluble in water and insoluble in kerosene and pertrol. 4. Conduction of electricity:ionic compounds in solid state-----does not conduct electricity. Reason—Ions can not move due to rigid solid structure. Ionic compounds conduct electricity in molten state. Reason-- Ions can move free ...
... 3. Solubility: soluble in water and insoluble in kerosene and pertrol. 4. Conduction of electricity:ionic compounds in solid state-----does not conduct electricity. Reason—Ions can not move due to rigid solid structure. Ionic compounds conduct electricity in molten state. Reason-- Ions can move free ...
Notes - Organization of Matter
... • Examples include: Water (H2O), Glucose or sugar (C6H12O6), and Table Salt (NaCl). ...
... • Examples include: Water (H2O), Glucose or sugar (C6H12O6), and Table Salt (NaCl). ...
The d-block elements are commonly known as transition
... Transition metal compounds are paramagnetic when they have one or more unpaired d electrons. Some compounds are diamagnetic. These include octahedral, lowspin, d6 and square-planar d8complexes. In these cases, crystal field splitting is such that all the electrons are paired up. Ferromagnetism occu ...
... Transition metal compounds are paramagnetic when they have one or more unpaired d electrons. Some compounds are diamagnetic. These include octahedral, lowspin, d6 and square-planar d8complexes. In these cases, crystal field splitting is such that all the electrons are paired up. Ferromagnetism occu ...
Electron Diffraction study of Layer Structures in La-Mg
... Iron, Cobalt or Nickel) by Kahn.4–6) Kahn pointed out that the structures could be regarded as a mixture of two structures, the Laves phase and the CaCu5 structure. Eelectron microscopic studies were performed by Komura et al. for the SmCo and Sm-Ni alloys.7–11) They reported that several complex st ...
... Iron, Cobalt or Nickel) by Kahn.4–6) Kahn pointed out that the structures could be regarded as a mixture of two structures, the Laves phase and the CaCu5 structure. Eelectron microscopic studies were performed by Komura et al. for the SmCo and Sm-Ni alloys.7–11) They reported that several complex st ...
Nickel-Titanium Memory Metal
... Because martensite is also slightly denser than austenite, by LeChatelier’s principle, which states that an increase in pressure favors the denser phase of multiple phases at equilibrium, pressure can be used to convert austenite to martensite. This is analogous to the pressure from ice skates helpi ...
... Because martensite is also slightly denser than austenite, by LeChatelier’s principle, which states that an increase in pressure favors the denser phase of multiple phases at equilibrium, pressure can be used to convert austenite to martensite. This is analogous to the pressure from ice skates helpi ...
Effect of SiC Grain Refining on Wear Resistance of Mg
... grain size measurements. After that the mold with remaining melt inside slowly lowered in to water to be solidified. Grain size measurements were conducted using line intercept method. Hardness tests were realized using a Vickers hardness tester, using 5 kg load and 30 s of loading duration. All giv ...
... grain size measurements. After that the mold with remaining melt inside slowly lowered in to water to be solidified. Grain size measurements were conducted using line intercept method. Hardness tests were realized using a Vickers hardness tester, using 5 kg load and 30 s of loading duration. All giv ...
New substances are formed by chemical reactions. When elements
... units which make up the compound. For example, in iron sulfide every iron atom is joined to one sulfur atom, so we show its formula as FeS. In sodium oxide, there are two sodium atoms for every oxygen atom, so we show its formula as Na2O. Notice that the 2 is written as a subscript, so Na2O would be ...
... units which make up the compound. For example, in iron sulfide every iron atom is joined to one sulfur atom, so we show its formula as FeS. In sodium oxide, there are two sodium atoms for every oxygen atom, so we show its formula as Na2O. Notice that the 2 is written as a subscript, so Na2O would be ...
Alloy

An alloy is a mixture of metals or a mixture of a metal and another element. Alloys are defined by metallic bonding character. An alloy may be a solid solution of metal elements (a single phase) or a mixture of metallic phases (two or more solutions). Intermetallic compounds are alloys with a defined stoichiometry and crystal structure. Zintl phases are also sometimes considered alloys depending on bond types (see also: Van Arkel-Ketelaar triangle for information on classifying bonding in binary compounds).Alloys are used in a wide variety of applications. In some cases, a combination of metals may reduce the overall cost of the material while preserving important properties. In other cases, the combination of metals imparts synergistic properties to the constituent metal elements such as corrosion resistance or mechanical strength. Examples of alloys are steel, solder, brass, pewter, duralumin, phosphor bronze and amalgams.The alloy constituents are usually measured by mass. Alloys are usually classified as substitutional or interstitial alloys, depending on the atomic arrangement that forms the alloy. They can be further classified as homogeneous (consisting of a single phase), or heterogeneous (consisting of two or more phases) or intermetallic.