4.8-Quantum Mechanics
... including why some spectral lines are brighter than others (some electron transitions are more likely to occur so with a large number of atoms, there are more atoms emitting that wavelength) •The duality of matter makes it impossible to develop a set of equations that tells us both exactly where a ...
... including why some spectral lines are brighter than others (some electron transitions are more likely to occur so with a large number of atoms, there are more atoms emitting that wavelength) •The duality of matter makes it impossible to develop a set of equations that tells us both exactly where a ...
Electronic structure_(download)
... The missing link in Bohr’s model was the quantum nature of the electron Quantum mechanics yields a viable model for the electrons in all the elements The extent to which it is real or simply an abstraction remains a fascinating, complex and unresolved argument ...
... The missing link in Bohr’s model was the quantum nature of the electron Quantum mechanics yields a viable model for the electrons in all the elements The extent to which it is real or simply an abstraction remains a fascinating, complex and unresolved argument ...
Concept of the Gibbsian ensemble
... Transition from classical to quantum statistics In classical mechanics a state of a system is determined by knowledge of position, q, and momentum, p. Dynamic evolution given by : trajectory in -space ...
... Transition from classical to quantum statistics In classical mechanics a state of a system is determined by knowledge of position, q, and momentum, p. Dynamic evolution given by : trajectory in -space ...
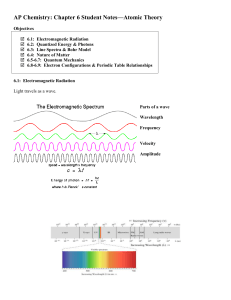
Ch. 6 notes
... 6.5-6.7: Quantum Mechanics Developed by Werner Heisenberg (1901-1976), Louis De Broglie (1892-1987), Erwin Schrodinger (1887-1961) This answers the question: Where is the _____________ in the atom? The answer is complex. We can’t say exactly where the atom is. We can only say where we think it _____ ...
... 6.5-6.7: Quantum Mechanics Developed by Werner Heisenberg (1901-1976), Louis De Broglie (1892-1987), Erwin Schrodinger (1887-1961) This answers the question: Where is the _____________ in the atom? The answer is complex. We can’t say exactly where the atom is. We can only say where we think it _____ ...
the squared modulus of the wave function is the probability density
... The good news is that the Schroedinger equation for the hydrogen atom has an EXACT ANALYTICAL solution! (this is one of the few problems in Quantum Mechanics that does have such a solution – most problems in QM cannot be solved exactly). The bad news, however, is that the procedure of solving the eq ...
... The good news is that the Schroedinger equation for the hydrogen atom has an EXACT ANALYTICAL solution! (this is one of the few problems in Quantum Mechanics that does have such a solution – most problems in QM cannot be solved exactly). The bad news, however, is that the procedure of solving the eq ...
107 chem Assement Q
... c. fundamental state. d. original state. 5. The hydrogen emission spectrum includes light with a wavelength of 434 nanometers. This is caused by an electron moving from: a. the n = 3 state to the n = 2 state. b. the n = 4 state to the n = 2 state. c. the n = 5 state to the n = 2 state. d. the n = 6 ...
... c. fundamental state. d. original state. 5. The hydrogen emission spectrum includes light with a wavelength of 434 nanometers. This is caused by an electron moving from: a. the n = 3 state to the n = 2 state. b. the n = 4 state to the n = 2 state. c. the n = 5 state to the n = 2 state. d. the n = 6 ...
PhD position: Quantum information processing with single electron spins
... which are intractable with other types of computer. It is natural to use the spin of an electron as a quantum bit because spin down is ‘0’, spin up is ‘1’, and all possible superposition states can be accessed with magnetic resonance. Nitrogen-vacancy (NV) centres in diamond have isolated electrons ...
... which are intractable with other types of computer. It is natural to use the spin of an electron as a quantum bit because spin down is ‘0’, spin up is ‘1’, and all possible superposition states can be accessed with magnetic resonance. Nitrogen-vacancy (NV) centres in diamond have isolated electrons ...
Please look over the following review questions
... quite different from the radius predicted by Bohr that agrees with the orbital radius of Bohr ...
... quite different from the radius predicted by Bohr that agrees with the orbital radius of Bohr ...
Quantum electrodynamics

In particle physics, quantum electrodynamics (QED) is the relativistic quantum field theory of electrodynamics. In essence, it describes how light and matter interact and is the first theory where full agreement between quantum mechanics and special relativity is achieved. QED mathematically describes all phenomena involving electrically charged particles interacting by means of exchange of photons and represents the quantum counterpart of classical electromagnetism giving a complete account of matter and light interaction.In technical terms, QED can be described as a perturbation theory of the electromagnetic quantum vacuum. Richard Feynman called it ""the jewel of physics"" for its extremely accurate predictions of quantities like the anomalous magnetic moment of the electron and the Lamb shift of the energy levels of hydrogen.