LOYOLA COLLEGE (AUTONOMOUS), CHENNAI – 600 034
... 5. What is a Green’s function? 6. What is screened Coulomb potential? 7. Distinguish between first and second order transitions of the time dependent perturbation theory with the help of schematic diagrams. 8. What is dipole approximation in emission/absorption process of an atom? 9. What is the lim ...
... 5. What is a Green’s function? 6. What is screened Coulomb potential? 7. Distinguish between first and second order transitions of the time dependent perturbation theory with the help of schematic diagrams. 8. What is dipole approximation in emission/absorption process of an atom? 9. What is the lim ...
Postulate 1 of Quantum Mechanics (wave function)
... • The wavefunction must be single-valued, continuous, finite (not infinite over a finite range), and normalized (the probability of find it somewhere is 1). ...
... • The wavefunction must be single-valued, continuous, finite (not infinite over a finite range), and normalized (the probability of find it somewhere is 1). ...
Physics 30 Atomic Model Review
... A photon of visible light with wavelength of 435nm is emitted from a hydrogen atom when an electron moves from a certain energy level to the second energy level. a. What is the Energy of this photon? (2) ...
... A photon of visible light with wavelength of 435nm is emitted from a hydrogen atom when an electron moves from a certain energy level to the second energy level. a. What is the Energy of this photon? (2) ...
An X-ray photon of wavelength 6 pm (1 pm = 10^-12 m
... an electron, so that the scattered photon goes in a direction opposite to that of the incident photon. The electron is initially at rest. (a) How much longer is the wavelength of the scattered photon than that of the incident photon? (b) What is the kinetic energy of the recoiling electron? (Use the ...
... an electron, so that the scattered photon goes in a direction opposite to that of the incident photon. The electron is initially at rest. (a) How much longer is the wavelength of the scattered photon than that of the incident photon? (b) What is the kinetic energy of the recoiling electron? (Use the ...
visible Ultra violet Infra red Longer line ? Energy? Wavelength
... Bohr model cannot be correct! ...
... Bohr model cannot be correct! ...
Quantum Mechanical Scattering using Path Integrals
... Undergraduate Colloquium in Mathematics Thursday, February 2nd 2PM-3PM STV 325 ...
... Undergraduate Colloquium in Mathematics Thursday, February 2nd 2PM-3PM STV 325 ...
Quantum Theory and Electrons as Waves
... If light could have particle-like behavior, then could matter have wave-like behavior? ...
... If light could have particle-like behavior, then could matter have wave-like behavior? ...
LOYOLA COLLEGE (AUTONOMOUS), CHENNAI – 600 034
... 15. The internuclear distance of the 1H35Cl molecule is 0.128 nm. Calculate the spacing of the lines in its rotational spectrum in terms of cm-1. 16. Obtain all the possible term symbols for a 4F state. ...
... 15. The internuclear distance of the 1H35Cl molecule is 0.128 nm. Calculate the spacing of the lines in its rotational spectrum in terms of cm-1. 16. Obtain all the possible term symbols for a 4F state. ...
PHYSICS 215 - Thermodynamics and Modern Physics Name:
... Anomalous Zeeman Effect: VB = µBBgmj where g = Landé g-factor = 1 + J(J+1)+S(S+1)-L(L+1) ...
... Anomalous Zeeman Effect: VB = µBBgmj where g = Landé g-factor = 1 + J(J+1)+S(S+1)-L(L+1) ...
Unit 1: Kinematics - Pre University Courses
... Louis de Broglie believed that all entities have wave-like properties but these properties are only significant and measureable for tiny, fast-moving particles like the electron. Erwin Schrödinger imagined electron behaviour within the atom structure as a wave phenomenon, described by a wave mechani ...
... Louis de Broglie believed that all entities have wave-like properties but these properties are only significant and measureable for tiny, fast-moving particles like the electron. Erwin Schrödinger imagined electron behaviour within the atom structure as a wave phenomenon, described by a wave mechani ...
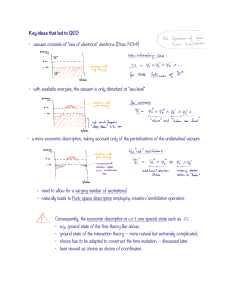
Key ideas that led to QED vacuum consists of "sea of electrons
... Many symbolic manipulations have to conducted on the integrant such as: • normal ordering due to the infinitely many charges in the Dirac sea; • mass renormalization due to the ultraviolet/infrared divergence of the photon field; • charge renormalization, again due to the infinitely many charges in ...
... Many symbolic manipulations have to conducted on the integrant such as: • normal ordering due to the infinitely many charges in the Dirac sea; • mass renormalization due to the ultraviolet/infrared divergence of the photon field; • charge renormalization, again due to the infinitely many charges in ...
Practice Problem Set #6
... 1. Traffic signals are often now made of LEDs (light-emitting diodes). The light from an amber signal has a wavelength of 595 nm, and that from a green signal has wavelength of 500 nm. Which has the higher frequency? Which has the highest energy per photon? Calculate the frequency of amber light. 2. ...
... 1. Traffic signals are often now made of LEDs (light-emitting diodes). The light from an amber signal has a wavelength of 595 nm, and that from a green signal has wavelength of 500 nm. Which has the higher frequency? Which has the highest energy per photon? Calculate the frequency of amber light. 2. ...
WAVE MECHANICS (Schrödinger, 1926)
... WAVE MECHANICS * The energy depends only on the principal quantum number, as in the Bohr model: En = -2.179 X 10-18J /n2 * The orbitals are named by giving the n value followed by a letter symbol for l: l= 0,1, 2, 3, 4, 5, ... s p d f g h ... * All orbitals with the same n are called a “shell”. All ...
... WAVE MECHANICS * The energy depends only on the principal quantum number, as in the Bohr model: En = -2.179 X 10-18J /n2 * The orbitals are named by giving the n value followed by a letter symbol for l: l= 0,1, 2, 3, 4, 5, ... s p d f g h ... * All orbitals with the same n are called a “shell”. All ...
Word Format
... Many lines were not seen. This indicated that there were selection rules that determined what lines were present. ...
... Many lines were not seen. This indicated that there were selection rules that determined what lines were present. ...
Notes27and29January2014BasicQuantumMechanics
... Quantum Theory for Semiconductors How to determine the behavior of electrons in the semiconductor? • Mathematical description of motion of electrons in quantum mechanics ─ Schrödinger’s Equation • Solution of Schrödinger’s Equation energy band structure and probability of finding a electron at a pa ...
... Quantum Theory for Semiconductors How to determine the behavior of electrons in the semiconductor? • Mathematical description of motion of electrons in quantum mechanics ─ Schrödinger’s Equation • Solution of Schrödinger’s Equation energy band structure and probability of finding a electron at a pa ...
CHM 50- Class activity
... The retina of a human eye can detect light when radiant energy incident on it is at least 4.0 x 10-17 J. For light of 5.85 nm wavelength , how many photons does this energy correspond to? ...
... The retina of a human eye can detect light when radiant energy incident on it is at least 4.0 x 10-17 J. For light of 5.85 nm wavelength , how many photons does this energy correspond to? ...
Quantum electrodynamics

In particle physics, quantum electrodynamics (QED) is the relativistic quantum field theory of electrodynamics. In essence, it describes how light and matter interact and is the first theory where full agreement between quantum mechanics and special relativity is achieved. QED mathematically describes all phenomena involving electrically charged particles interacting by means of exchange of photons and represents the quantum counterpart of classical electromagnetism giving a complete account of matter and light interaction.In technical terms, QED can be described as a perturbation theory of the electromagnetic quantum vacuum. Richard Feynman called it ""the jewel of physics"" for its extremely accurate predictions of quantities like the anomalous magnetic moment of the electron and the Lamb shift of the energy levels of hydrogen.