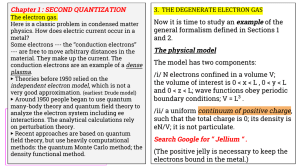
3. THE DEGENERATE ELECTRON GAS Chapter 1 : SECOND QUANTIZATION
... This is the limit N → ∞, V → ∞, with n = N/V constant and finite. As we go along we’ll make approximations that are valid in this limit. ...
... This is the limit N → ∞, V → ∞, with n = N/V constant and finite. As we go along we’ll make approximations that are valid in this limit. ...
The positron
... same mass but opposite charge. • Every particle has such an antiparticle. Some are identical to their antiparticle, for example the photon. • A positron can be compared to a hole in a semiconductor, which is an electron missing from an occupied band (Lect. ...
... same mass but opposite charge. • Every particle has such an antiparticle. Some are identical to their antiparticle, for example the photon. • A positron can be compared to a hole in a semiconductor, which is an electron missing from an occupied band (Lect. ...
Introduction to Chemistry
... Learning Objectives (from Zumdahl Resource Guide): (3-4 days lecture/discussion) To characterize electromagnetic radiation in terms of wavelength, frequency, and speed. To introduce the concept of quantized energy. To show that light has both wave and particulate properties. To describe how diffract ...
... Learning Objectives (from Zumdahl Resource Guide): (3-4 days lecture/discussion) To characterize electromagnetic radiation in terms of wavelength, frequency, and speed. To introduce the concept of quantized energy. To show that light has both wave and particulate properties. To describe how diffract ...
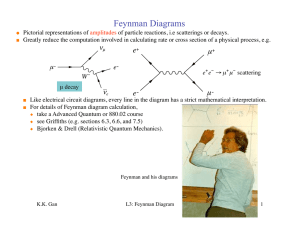
Lecture 1 - Particle Physics Group
... Given the list of particles and vertices which exist in a certain theory, (e.g. the SM) we can use FDs to find out all the processes which are allowed by the theory, and make rough estimates of their relative probability. Every vertex and particle corresponds to a term in the Lagrangian (the formula ...
... Given the list of particles and vertices which exist in a certain theory, (e.g. the SM) we can use FDs to find out all the processes which are allowed by the theory, and make rough estimates of their relative probability. Every vertex and particle corresponds to a term in the Lagrangian (the formula ...
Baby-Quiz
... don’t all the photoelectrons have the same kinetic energy when they leave the metal’s surface? 4. What property of the emitted electrons depends on the intensity of incident light?What property of the emitted photoelectrons depends on the frequency of incident light? ...
... don’t all the photoelectrons have the same kinetic energy when they leave the metal’s surface? 4. What property of the emitted electrons depends on the intensity of incident light?What property of the emitted photoelectrons depends on the frequency of incident light? ...
WAVE MECHANICS AND QUANTUM NUMBERS
... 2. supported by the facts that electrons undergo diffraction and interference 3. Werner Heisenberg 1927- Heisenberg Uncertainty Principle: it is impossible to simultaneously identify the position and velocity of an electron, or any particle. 4. wave mechanics looks to suggest the locations of electr ...
... 2. supported by the facts that electrons undergo diffraction and interference 3. Werner Heisenberg 1927- Heisenberg Uncertainty Principle: it is impossible to simultaneously identify the position and velocity of an electron, or any particle. 4. wave mechanics looks to suggest the locations of electr ...
CH1710 HW#7 (2017)-Quanta, electron config
... 4. Consider the photon absorbed when a hydrogen electron undergoes a transition from n=1 to n=3. a) Use the Rydberg equation to calculate the wavelength (in Å) of this photon. ...
... 4. Consider the photon absorbed when a hydrogen electron undergoes a transition from n=1 to n=3. a) Use the Rydberg equation to calculate the wavelength (in Å) of this photon. ...
Powerpoint handout
... Niels Bohr explained all the various lines by proposing that electrons in atoms could have only certain energies, and that light was given off when an electron underwent a transition from a higher energy level to a lower one. ...
... Niels Bohr explained all the various lines by proposing that electrons in atoms could have only certain energies, and that light was given off when an electron underwent a transition from a higher energy level to a lower one. ...
Lecture #3
... A moving “particle” is described by a superposition of a great many sin and cos waves which constructively interfere to give a Gaussian probability near a certain point but destructively interfere everywhere else. The Gaussian “wave packet” moves according to the kinetic energy given by the average ...
... A moving “particle” is described by a superposition of a great many sin and cos waves which constructively interfere to give a Gaussian probability near a certain point but destructively interfere everywhere else. The Gaussian “wave packet” moves according to the kinetic energy given by the average ...
Chemistry 1 Concept 5 “Electrons in Atoms” Study Guide
... 3. The energy of a photon is related to its ______________ 4. Give the number of orbitals for each sublevel: s _____, p ______, d ______, f ______ 5. What is the wavelength of microwaves of 4.0 x 109 Hz frequency? _______________ 6. An electron for which n = 4 has more _______ than an electron for w ...
... 3. The energy of a photon is related to its ______________ 4. Give the number of orbitals for each sublevel: s _____, p ______, d ______, f ______ 5. What is the wavelength of microwaves of 4.0 x 109 Hz frequency? _______________ 6. An electron for which n = 4 has more _______ than an electron for w ...
Atomic Structure and Quantum Theory
... Planck and Blackbody Radiation Einstein and the Photoelectric Effect Spectra Quantization ...
... Planck and Blackbody Radiation Einstein and the Photoelectric Effect Spectra Quantization ...
Quantum electrodynamics

In particle physics, quantum electrodynamics (QED) is the relativistic quantum field theory of electrodynamics. In essence, it describes how light and matter interact and is the first theory where full agreement between quantum mechanics and special relativity is achieved. QED mathematically describes all phenomena involving electrically charged particles interacting by means of exchange of photons and represents the quantum counterpart of classical electromagnetism giving a complete account of matter and light interaction.In technical terms, QED can be described as a perturbation theory of the electromagnetic quantum vacuum. Richard Feynman called it ""the jewel of physics"" for its extremely accurate predictions of quantities like the anomalous magnetic moment of the electron and the Lamb shift of the energy levels of hydrogen.