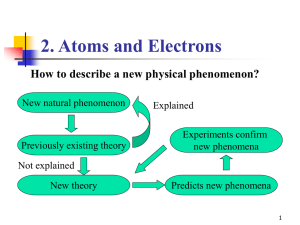
Section 3.7
... quantum mechanics. 7. Heisenberg would argue that the measured speed included uncertainty; but the amount of this uncertainty is infinitesimal for an object such as a car, certainly many orders of magnitude less than the precision of the “radar” gun. 8. Dr. Richard Bader researches the nature of ato ...
... quantum mechanics. 7. Heisenberg would argue that the measured speed included uncertainty; but the amount of this uncertainty is infinitesimal for an object such as a car, certainly many orders of magnitude less than the precision of the “radar” gun. 8. Dr. Richard Bader researches the nature of ato ...
Quantum dots and radio-frequency electrometers in silicon
... Cavendish Laboratory, University of Cambridge An important goal for solid-state quantum computing is to confine a single electron in silicon, then manipulate and subsequently determine its spin state. Silicon has a low nuclear spin density which, together with the low spin-orbit coupling in this mat ...
... Cavendish Laboratory, University of Cambridge An important goal for solid-state quantum computing is to confine a single electron in silicon, then manipulate and subsequently determine its spin state. Silicon has a low nuclear spin density which, together with the low spin-orbit coupling in this mat ...
Quantum numbers
... finding the electron at position 1 relative to position 2. If the number is 100, the electron is 100 times more likely to be in position 1 than 2. ...
... finding the electron at position 1 relative to position 2. If the number is 100, the electron is 100 times more likely to be in position 1 than 2. ...
The Hydrogen Atom
... The Bohr Model In 1913 Niels Bohr proposed a theory of the hydrogen atom that could account for its stability and for the frequencies of its spectral lines. • An electron can circle the nucleus without losing energy only in certain specific orbits. • The energy of the electron depends on which orbi ...
... The Bohr Model In 1913 Niels Bohr proposed a theory of the hydrogen atom that could account for its stability and for the frequencies of its spectral lines. • An electron can circle the nucleus without losing energy only in certain specific orbits. • The energy of the electron depends on which orbi ...
Advanced Quantum Mechanics Syllabus and Introduction
... is after all, first order in time and second order in space coordinates. This is not too hard to deal with. We use the Heisenberg picture, so that space and time coordinates appear together in the operators. We also need new wave equations, which depend on the spin of particles involved. The second ...
... is after all, first order in time and second order in space coordinates. This is not too hard to deal with. We use the Heisenberg picture, so that space and time coordinates appear together in the operators. We also need new wave equations, which depend on the spin of particles involved. The second ...
4.2 The Quantum Model of the Atom Vocab Electromagnetic
... - A state in which an atom has more energy than it does at its ground state. Emission-Line Spectrum - A diagram or graph that indicates the degree to which a substance emits radiant energy with respect to wavelength. Continuous Spectrum The uninterrupted broad band of all colors [wavelengths] emitte ...
... - A state in which an atom has more energy than it does at its ground state. Emission-Line Spectrum - A diagram or graph that indicates the degree to which a substance emits radiant energy with respect to wavelength. Continuous Spectrum The uninterrupted broad band of all colors [wavelengths] emitte ...
Quantum Theory 1 - Home Exercise 4
... (a) Find the normalized stationary states of the system and explicitly show that they form an orthonormal basis. (b) Calculate the dispersion relation ωn (kn ) and show that ωn = ω−n . (c) Show that any linear combination of the stationary states is also a solution of (timeP dependant) Schroedinger’ ...
... (a) Find the normalized stationary states of the system and explicitly show that they form an orthonormal basis. (b) Calculate the dispersion relation ωn (kn ) and show that ωn = ω−n . (c) Show that any linear combination of the stationary states is also a solution of (timeP dependant) Schroedinger’ ...
Answer
... values from 0 to n − 1. In this case n = 2, so the allowed values of the angular momentum quantum number are 0 and 1. Each allowed value of the angular momentum quantum number labels a subshell. Within a given subshell (label l) there are 2l + 1 allowed energy states (orbitals) each labeled by a dif ...
... values from 0 to n − 1. In this case n = 2, so the allowed values of the angular momentum quantum number are 0 and 1. Each allowed value of the angular momentum quantum number labels a subshell. Within a given subshell (label l) there are 2l + 1 allowed energy states (orbitals) each labeled by a dif ...
QM-01
... Consider our attempt to view the electrons in the double slit system by shining light of wavelength λ on them with photon momentum pphoton = h/λ . If we manage to see an electron it will be because one of these photons has struck it. Clearly the electron momentum will be affected by this interaction ...
... Consider our attempt to view the electrons in the double slit system by shining light of wavelength λ on them with photon momentum pphoton = h/λ . If we manage to see an electron it will be because one of these photons has struck it. Clearly the electron momentum will be affected by this interaction ...
1.1 What has to be explained by Quantum mechanics?
... But ”free” and ”occupied” states within a band, sizes of band gaps, etc. classify metals, semiconductors, and insulators. • Why, in contrast, must photons be Bosons?!? (One single QM state macroscopically measurable) • What is: Schrödinger equation, Operator, commutator, probability function, wave ...
... But ”free” and ”occupied” states within a band, sizes of band gaps, etc. classify metals, semiconductors, and insulators. • Why, in contrast, must photons be Bosons?!? (One single QM state macroscopically measurable) • What is: Schrödinger equation, Operator, commutator, probability function, wave ...
Document
... a photon in a given region of space at an instant of time is proportional to the number N of photons per unit volume V at that time which its proportional to the intensity of the radiation I or the Square of the electric field amplitude E. ...
... a photon in a given region of space at an instant of time is proportional to the number N of photons per unit volume V at that time which its proportional to the intensity of the radiation I or the Square of the electric field amplitude E. ...
Quantum electrodynamics

In particle physics, quantum electrodynamics (QED) is the relativistic quantum field theory of electrodynamics. In essence, it describes how light and matter interact and is the first theory where full agreement between quantum mechanics and special relativity is achieved. QED mathematically describes all phenomena involving electrically charged particles interacting by means of exchange of photons and represents the quantum counterpart of classical electromagnetism giving a complete account of matter and light interaction.In technical terms, QED can be described as a perturbation theory of the electromagnetic quantum vacuum. Richard Feynman called it ""the jewel of physics"" for its extremely accurate predictions of quantities like the anomalous magnetic moment of the electron and the Lamb shift of the energy levels of hydrogen.